ARTICLE
Successful treatment of aspiration pneumonia in a juvenile raccoon (Procyon lotor)
Veronica Gordon
PAWS Wildlife Center, Snohomish, WA, USA
Abstract
A healthy orphaned raccoon (Procyon lotor) admitted to the PAWS Wildlife Center in July 2023 suffered from aspiration pneumonia while in rehabilitative care. The patient experienced depressed mentation and severe respiratory distress immediately following aspiration. Resolution of symptoms consistent with pneumonia occurred within 2 weeks of the date of aspiration through conservative management. This case report will discuss successful management of severe aspiration pneumonia following administration of formula via gavage feeding in a juvenile raccoon.
Keywords
Aspiration, Raccoon (Procyon lotor), Aspiration pneumonia, V/Q mismatch, nebulization
Citation: Wildlife Rehabilitation Bulletin 2024, 42(2), 60–65, http://dx.doi.org/10.53607/wrb.v42.290
Copyright: Wildlife Rehabilitation Bulletin 2024. © 2024 V. Gordon. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Accepted: 3 November 2024; Published: 13 December 2024
Competing interests and funding: The author reports no conflict of interest.
Correspondence: Veronica Gordon, PAWS Wildlife Center, 13508 SR-9 SE, Snohomish, WA 98296. E-mail: vgordon@paws.org
Introduction
In the course of rehabilitating orphaned and injured wildlife, the act of aspiration can be a potential risk with the regular administration of enteral nutrition. At the time of this article, the occurrence of aspiration and frequency at which it results in more severe disease in wildlife is not well documented in scientific literature. Aspiration itself can be the result of many things: dysphagia, emesis, and regurgitation being among the most typical differentials. In a 2019 study evaluating clinical and radiographic findings in cats with aspiration pneumonia, “Potential predisposing conditions included: vomiting (12 of 28; 43%), anesthesia (5 of 28; 18%), enteral nutrition (5 of 28; 18%), preexisting esophageal disease (4 of 28; 14%), neurologic disease (2 of 28; 7%) and laryngeal disease (1 of 28; 3.6%); 15 of 28 (53%) had more than one potentially predisposing condition” (Levy et al. 2019).
Aspiration pneumonia and aspiration pneumonitis are two prevalent escalations from aspiration. The severity of the outcome of aspiration is contingent upon several factors: the material inhaled, the type of bacteria inhaled, and the distribution of foreign material in the lungs (Kuehn 2018). Aspiration pneumonia is defined as a bacterial infection in the lungs caused by the inhalation of foreign material; its definition is centered around the presence of infection (Kuehn 2018). Aspiration pneumonitis still has most of the same physiologic disturbances, but instead causes inflammation without subsequent development of a bacterial infection (Teramoto 2020).
Background and case report
There is a unique opportunity to observe and affirm the incidence of aspiration during administration of enteral nutrition in a wildlife rehabilitation setting. In a retrospective study comparing human and veterinary cases of aspiration pneumonia and pneumonitis, the authors acknowledged that as the origin of aspiration is not often identified it becomes a diagnosis of presumption (Sherman & Karagiannis 2017). In the case of this otherwise healthy juvenile raccoon (Procyon lotor), we were fortunate to be allowed quick action in response to having a known incident of aspiration after the patient received a gavage administration of formula. Directly after the incident, the patient experienced depressed mentation and severe respiratory distress. On physical examination, the patient was noted to have pale mucous membranes, shallow breathing, and distinct increased noise on inspiration and expiration via auscultation of the lung fields.
After the incident of aspiration, the patient was immediately placed in an oxygen-enriched chamber. When aspiration occurs, the ability to breathe in fully is often inhibited partly due to alveolar surfactant disruption causing increased surface tension and alveolar collapse (Cooper 2020). An awake dorsoventral diagnostic plop radiograph (Fig. 1) showed that a severe diffuse interstitial to alveolar pattern was obscuring nearly all of the left lung fields and the majority of the right lung fields. As a consequence of regurgitating aspirated stomach content, gastric acid causes chemical burn to the bronchial and alveolar epithelial cells as well as the introduction of food particles to the trachea and lungs. The patient was given meloxicam to address associated inflammation as well as terbutaline, a bronchodilator, subcutaneously 30 min after being placed in oxygen. In very acute stages of aspiration pneumonia, bronchodilators can be indicated. After an additional 30 min the patient’s mentation was brighter, and despite respiratory rate being increased, respirations were of greater depth and less effort.
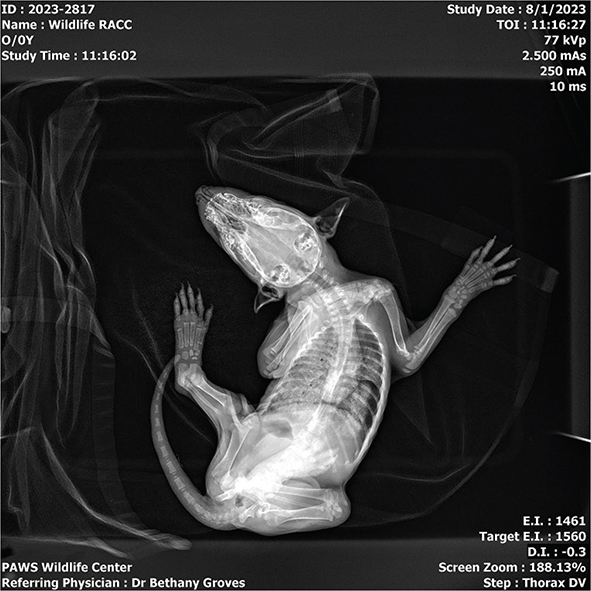
Fig. 1 Plop dorsoventral (DV) radiograph taken immediately post-aspiration.
Paired with the lungs reduced ability to clear bacteria and debris, the disruption of normal alveolar surfactant compromises the antimicrobial properties that normally protect the alveoli (Iradufasha 2019). As the respiratory system’s ability to fight infection is significantly challenged after aspiration, antibiotic therapy in the form of both Clavamox™ (amoxicillin trihydrate, clavulanate potassium; Zoetis Inc, Parsippany, NJ) and enrofloxacin was given orally within the first hour of critical patient management in this case. Medical management in its entirety included broad-spectrum antibiotic coverage twice daily for 14 days, meloxicam once daily for 7 days, subcutaneous crystalloid fluid bolus twice daily for 3 days and terbutaline twice daily for the first 24 hr.
Nebulization therapy was also employed in conjunction with oxygen therapy by utilizing 3% sterile hypertonic saline. Nebulization allows small liquid particles to potentially enhance clearance of secretions as “adequate hydration is essential to facilitate clearance of respiratory exudates” (Dear 2019). Nebulization therapy was performed for 20 min increments followed by physiotherapy in the form of coupage every 12 hr for the first 48 hr. When regurgitation is not of concern, chest physiotherapy in dogs has resulted in a significant improvement of hypoxemia within the first 48 hr of care, subsequently reducing the amount of time those patients spent in oxygen (Pouzot-Nevoret et al. 2021).
Additional gavage feedings of formula were suspended for 7 hr on the day of the incident to allow for stabilization. After stabilization, enteral nutrition continued at normal increments with careful consideration of the animal’s condition. After 72 hr, the patient had returned to normal mentation. The respiratory pattern appeared much improved despite respirations presenting as shallow and abrupt; no forced effort was appreciated but an abdominal component was noted. On auscultation, there were no crackles or wheezes, though a rale could be heard. The decision was made to trial the patient off of oxygen supplementation after 72 hr; however, several hours after oxygen was removed, the patient was found to have equivocally worsened respiratory effort. The presence of clinical signs such as respiratory rate, effort, and perceived work of respirations are the guiding factors for providing and determining the length of supplemental oxygen therapy (Cooper 2020). The patient was subsequently kept on supplemental oxygen therapy overnight and discontinued the following day with success. Fig. 2 shows the radiographic change to the lungs 4 days post-aspiration.

Fig. 2 Recheck plop DV radiograph exhibiting lung field.
After 1 week of treatment, the patient exhibited no grossly audible respiratory noise, and the respiratory rate had returned to normal. On auscultation, the patient had no crackles, wheezes, or rales, but did have increased bronchovesicular sounds diffusely throughout all lung fields. Following 2 weeks post-aspiration, the patient’s energy, exercise tolerance, and auscultation were normal. Recheck radiographs performed under chemical sedation 18 days post-aspiration showed increased fluid/soft tissue opacity still present in the region of the right middle lung but were otherwise normal (Figs. 3, 4). The patient was subsequently cleared from further medical care and released into the wild 2 months later after pre-release conditioning and assessment proved the patient nearly indistinguishable from its cagemates. Pre-release conditioning for juvenile raccoons involves staging animals into larger enclosures with conspecifics and daily novel foraging enrichment.

Fig. 3 Sedated DV radiograph of lung fields: Sedated R lateral radiograph of lung fields.

Fig. 4 Sedated R lateral radiograph of lung fields.
Discussion
It should be of note that the successful management of this case was in large part due to the quick communication of the incident of aspiration. As with most wildlife rehabilitation centers, aspiration is not an everyday occurrence at PAWS but is either speculated or known to occur with some regularity. As previously stated, the patients’ decline from aspiration was a rapid progression. The pulmonary dysfunction and hypoxemia that occur as a consequence of aspiration results in hyperventilation as well as hypercapnia.
Oxygen is an excellent immediate therapy choice in the context of wildlife rehabilitation with consideration to the physiological strain aspiration places on a patient. It is often the most accessible low-risk critical treatment with regard to respiratory distress. Oxygen can be supplemented at 40–60%, confirmed through the use of an oxygen sensor, until respiratory difficulty lessens and the animal is amenable to weaning off of supplemental oxygen (Dear 2019). As in our case, the patient’s overt response to discontinuation of oxygen is often the most reliable indicator of need due to a lack of access to advanced diagnostic tools in wildlife rehabilitation. The author acknowledges that in other facets of veterinary medicine, there are several advanced diagnostic tools that can be used to better assess how well the body is oxygenating.
The length of administration of bronchodilators in this case was one that could likely be reduced in future cases, as the potential for bronchoconstriction is usually reduced to the first few hours after aspiration occurs. Within the first 2 hr of chemical burn damage to the epithelial cells of the airway, inflammatory mediators are released that promote bronchoconstriction and fluid accumulation in the interstitial and alveolar lung space (Cooper 2020). Conversely, bronchodilators can negatively impact the ventilation-perfusion (V/Q) ratio of the lungs if they are administered outside of the window of bronchoconstriction. Furosemide administration was considered as it can act as a bronchodilator as well, though ultimately was decided against in this case. Furosemide use was discouraged directly for use in aspiration pneumonia cases in one of our referenced literatures, “furosemide should not be used because drying of secretions traps material in the lower airway and perpetuates infection” (Dear 2013).
The V/Q ratio “is a measure of the relationship between the amount of air entering the alveoli (V) and the amount of blood flowing through the capillaries surrounding the alveoli in the lungs (Q)” (McIntire & Ling 2018). Unfortunately, due to the nature of aspiration itself the V/Q ratio is already under duress. Attempting to use bronchodilators outside of the initial period of bronchoconstriction carries the risk of further negatively challenging the balance of the V/Q ratio. Without advanced diagnostics to inform management, it is unknown what impact administering terbutaline for 24 hr may have had on our case.
In this case, combination therapy with broad-spectrum antibiotics was initiated without the benefit of culture and sensitivity testing. The use of culture and sensitivity testing is still strongly considered a primary diagnostic protocol in most applications of veterinary medicine (Pendergrass 2017). The practicality of this protocol can be challenged by the resource- and time-driven limitations at wildlife rehabilitation facilities, though it should still be of consideration, especially in cases where the exact incident of aspiration is unknown. Gram-negative enterics have been shown to be the most common representation of bacterial populations in cases of aspiration pneumonia, though gram-positive organisms are also often present (Cooper 2020).
The prevalence for Gram-negative bacteria, necessity for good penetration into the lung tissue, and resource challenges in wildlife medicine can make fluroquinolones the most advantageous antibiotic therapy choice for cases of suspected aspiration pneumonia. Special consideration should be given to the legality of utilizing fluroquinolones in potential food animals, as what species qualify as a food animal can vary significantly throughout the U.S. The author strongly recommends utilizing the NWRA Wildlife Formulary in conjunction with licensed veterinarians to determine appropriate drug dosages.
Conclusion
This case of aspiration pneumonia in an otherwise healthy juvenile raccoon was managed with a successful outcome using conservative medical management principles derived from domestic small animal veterinary practices. Additional cases in which this protocol is utilized are needed to better evaluate efficacy and outcome. Treatment for aspiration pneumonia is largely geared towards managing respiratory compromise, treating for secondary bacterial infections, identifying and addressing predisposing factors, and providing supportive care (Cooper 2020). The knowledge of the occurrence, severity of aspiration, and the immediacy of recognition are important factors in the successful management of these cases. Early intervention in the form of oxygen therapy, nebulization, antibiotics, NSAIDs, and, in limited cases, bronchodilators can help reduce the severity of aspiration-related disease as hours can make a difference.
Acknowledgments
The author would like to acknowledge the hard work of the PAWS Wildlife Center staff in rehabilitating this animal. The author would specifically like to thank Dr Nicki Rosenhagen and Dr Bethany Groves for their support of this paper and dedicated management of this patient.
References
| Cooper E. 2020. Clinical approach to aspiration pneumonia: evidence and opportunities. VIN/IVECCS, September. Accessed on the internet at https://www.vin.com/doc/?id=9745260 on 1 February 2024. |
| Dear J.D. 2019. Bacterial pneumonia in dogs and cats. Veterinary Clinics of North America: Small Animal Practice 50(2), 447–465, http://dx.doi.org/10.1016%2Fj.cvsm.2019.10.007 |
| Dear J.D. 2013. Bacterial pneumonia in dogs and cats. Veterinary Clinics of North America: Small Animal Practice, 44(1),143–159. http://dx.doi.org/10.1016/j.cvsm.2013.09.003 21 November 2013 |
| Iradufasha E. 2019. Osmosis: alveolar surface tension and surfactant. Philadelphia, PA: Elsevier. Accessed on the internet at https://www.osmosis.org/learn/Alveolar_surface_tension_and_surfactant on 16 February 2024. |
| Kuehn N. 2018. Pneumonia in dogs. Merck veterinary manual. Rahway, NJ: Merck & Co, Inc. Accessed on the internet at https://www.merckvetmanual.com/dog-owners/lung-and-airway-disorders-of-dogs/pneumonia-in-dogs on 16 February 2024. |
| Levy N., Ballegeer E. & Koenigshof A. 2019. Clinical and radiographic findings in cats with aspiration pneumonia: retrospective evaluation of 28 cases. Journal of Small Mammal Practice 60(6), 356–360. https://doi.org/10.1111/jsap.12990 |
| McIntire G. & Ling, J. 2018. Osmosis: Ventilation-perfusion ratios and V/Q mismatch. Philadelphia, PA: Elsevier. Accessed on the internet at https://www.osmosis.org/learn/Ventilation-perfusion_ratios_and_VQ_mismatchon 16 February 2024. |
| Pendergrass J. 2017. Aspiration pneumonia in pets and people. DVM 360. Accessed on the internet at https://www.dvm360.com/view/aspiration-pneumonia-in-pets-and-people on 20 February 2024. |
| Pouzot-Nevoret C., Magnin M., Barthélemy A., Goy-Thollot I., Cambournac M., Nectoux A. & Allaouchiche B. 2021. Effectiveness of coupage physiotherapy in dogs using passive slow expiratory techniques in dogs with airway fluid accumulation: a randomized controlled trial. Journal of Veterinary Internal Medicine 35(3), 1525–1535. https://doi.org/10.1111/jvim.16088 |
| Sherman R. & Karagiannis M. 2017. Aspiration pneumonia in the dog: A review. Topics in Companion Animal Medicine 32(1), 1–7. https://doi.org/10.1053/j.tcam.2017.05.003 |
| Teramoto, S. 2020. Animal models of aspiration pneumonia. In S. Teramoto & K. Komiya (eds.): Aspiration pneumonia. Respiratory Disease Series: Diagnostic Tools and Disease Managements. Pp. 143–151. Singapore: Springer. |