CASE REPORT
Case report: disseminated Staphylococcus aureus infections in two infant desert cottontail rabbits (Sylvilagus audubonii)
Gabriele C. Paul & Daniel G. Friend
Colorado Wild Rabbit Foundation, Erie, CO, USA
Abstract
Staphylococcus aureus is a frequent pathogen in mammals, including rabbits. It can cause suppurative inflammation with a variety of clinical presentations, sometimes resulting in high morbidity and mortality. Outbreaks have been reported in both domestic European rabbits (Oryctolagus cuniculus), and in cottontail rabbits (Sylvilagus spp.). The Colorado Wild Rabbit Foundation recently identified two cases of disseminated S. aureus infections in sibling infant desert cottontail rabbits (S. audubonii) that were presented for rehabilitation. This case report describes the course of the disease, attempted treatment, and pathology findings. Culture and sensitivity tests indicated susceptibility of the pathogen to all antibiotics tested, including trimethoprim-sulfamethoxazole (TMP-SMZ). However, both cases resulted in disseminated spread, against which TMP-SMZ was ineffective. Cottontail rabbit rehabilitators should be aware of the potentially subtle clinical signs, the possibility of outbreaks within a facility, and the difficulties in treating this disease.
Keywords
Abscesses; disseminated infection; rabbit diseases; trimethoprim-sulfamethoxazole; wildlife rehabilitation
Abbreviations
BID: twice a day
CWRF: Colorado Wild Rabbit Foundation
MIC: Minimum inhibitory concentration
MRSA: Methicillin-resistant Staphylococcus aureus
PO: oral
SID: once a day
TMP-SMZ: Trimethoprim-sulfamethoxazole
Citation: Wildlife Rehabilitation Bulletin 2024, 42(1), 10–16, http://dx.doi.org/10.53607/wrb.v42.273
Copyright: Wildlife Rehabilitation Bulletin 2024. © 2024 G.C. Paul and D.G. Friend. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Accepted: 28 January 2024; Published: 28 June 2024
Competing interests and funding: The authors report no conflict of interest.
The authors received no specific funding for this study.
Correspondence to: Gabriele C. Paul, Colorado Wild Rabbit Foundation, PO Box 1253, Erie, CO 80516, USA. E-mail: cowildrabbit@yahoo.com
Introduction
Staphylococcus aureus is a major pathogen that affects humans and other animals (Cheung et al. 2021; Stewart 2022). It is a well-known cause of morbidity and mortality in domestic European rabbits (Oryctolagus cuniculus), and infections have been found in several species of wild rabbits and hares (Barthold et al. 2016). While cases have been described in cottontail rabbits (Sylvilagus spp.) (McCoy & Steenbergen 1969; Wardyn et al. 2012), reports of infections in infant cottontail rabbits are rare. The Colorado Wild Rabbit Foundation (CWRF) recently identified two cases of S. aureus infections in sibling infant desert cottontail rabbits (S. audubonii) that were presented for rehabilitation. Here we provide a description of the course of the disease, attempted treatment, and pathology findings, from the wildlife rehabilitator’s perspective.
Background and case reports
Staphylococcus aureus is a gram-positive cocci bacterium that is a frequent inhabiter of the skin and other body sites of healthy rabbits; however, it can cause severe infections under some circumstances, such as when the pathogen gains entry into the body through wounds or other pathways (Cheung et al. 2021; Varga Smith 2023a). Many rabbits harbor the organism without ever becoming ill, so it may be considered part of their normal flora. In one study, S. aureus was isolated from 85% of samples collected from healthy pet rabbits (Jangsangthong et al. 2022). Hermans et al. (1999) tested rabbits from 19 rabbitries and found S. aureus in 11 out of 12 clinically healthy herds, as well as in seven out of seven unhealthy herds. Similarly, in a study of 400 intensively farmed rabbits in Italy, Attili et al. (2020) found that 71% were colonized with S. aureus at one or more body sites, but only 8.8% had lesions. These authors also found that the risk for S. aureus-infected skin lesions increased with age and number of body sites colonized.
Two patterns of infection can be attributed to different strains of the organism: low-level, sporadic lesions caused by low-virulence strains, and epizootic outbreaks with high morbidity and mortality caused by high-virulence strains (Hermans et al. 1999; Corpa et al. 2009; Barthold et al. 2016). Both virulence factors of the pathogen and host resistance play roles in causing disease (Spaulding et al. 2012; Varga Smith 2023a). Staphylococcus aureus can cause suppurative inflammation and has been known to result in a variety of diseases in rabbits, including dermatitis, mastitis, pododermatitis, multisystemic abscessation, respiratory infections, and septicemia (McCoy & Steenbergen 1969; Corpa et al. 2009; Varga Smith 2023a). According to these authors, it is spread primarily via direct contact, such as from a doe to her kits, bite wounds, or a shared environment. In commercial domestic rabbit production facilities, S. aureus-caused infections such as chronic abscesses, mastitis, and neonatal septicemia are well-known, and can be important causes of death in both adult and young rabbits (Corpa et al. 2009; Ferreira et al. 2014; Barthold et al. 2016).
Staphylococcus aureus grows well in culture, and gram-positive cocci in clusters can often be seen on gram stains of exudate (Markey et al. 2013), allowing for a rapid presumptive diagnosis (Ranzani et al. 2020). However, infections caused by this organism can be difficult to treat for a variety of reasons: they may result in chronic, encapsulated abscesses that are unable to heal unless surgically removed (Varga Smith 2023b); hematogenous spread can result in multiple abscesses throughout the body, including endocarditis, as well as septicemia (Spaulding et al. 2012; Stewart 2022; Varga Smith 2023a); even when susceptible, S. aureus infections can be resistant to treatment with antibiotics due to a number of virulence factors (Spaulding et al. 2012; Stewart 2022).
In July, 2023, two infant desert cottontail rabbits (approximately two weeks old), presumably siblings, were brought to a wildlife rehabilitation center in Colorado. They had been found hopping around in the open in a park during a Fourth of July fireworks display. Both appeared healthy at intake, with no injuries and in good body condition. They were not yet weaned, weighing 96 g (sib 1) and 86 g (sib 2). Reuniting the infants with their mother was not attempted because the nesting site could not be located; consequently, they were accepted for rehabilitation and transferred to the CWRF.
The rabbits were housed together and cared for using the standard CWRF infant rabbit protocols. After 2–3 days, both were readily eating milk replacer formula, initially from a syringe and then from a dish. They were also eating grass and various forbs and were steadily gaining weight. On day seven, sib 1 began showing non-specific signs of illness (Fig. 1), including a “puffed up” appearance with piloerection, a hunched posture, a small amount of discharge from both eyes, and the rabbit was favoring its right front paw. The next day, small pustules were noticed on the lower lip and chin (Fig. 2a). A subsequent examination revealed a swollen and inflamed right front paw (Fig. 2b), as well as an inflamed hind toe. As an infectious agent was then suspected, per the CWRF protocol, the rabbit was prescribed the antibiotic trimethoprim-sulfamethoxazole (TMP-SMZ, 40 mg/kg, PO, SID). While a common dose for TMP-SMZ is 15–30 mg/kg BID (Miller et al. 2017; Fisher & Graham 2018), Varga Smith (2023c) recommends 40 mg/kg BID. According to Budde & McCluskey (2023), the frequency of administration of TMP-SMZ is controversial, with most clinicians preferring twice-daily dosing. However, Fisher & Graham (2018) lists once per day dosing as an option for rabbits, albeit at 15–30 mg/kg. In order to minimize handling (and the associated stress) when treating wildlife, once daily drug dosing is generally preferred when possible. A pain medication (meloxicam, 1 mg/kg, PO, SID [Fisher & Graham 2018]) was also prescribed. Despite these treatments, the disease progressed rapidly. On day 10, the lower lip and both front feet were markedly swollen, and the overall status of the rabbit had declined to the point where the decision was made to euthanize.

Fig. 1 An infant desert cottontail rabbit (sib 1) with a disseminated Staphylococcus aureus infection, showing initial signs of illness, including piloerection, a hunched posture, and favoring its right front paw.

Fig. 2 An infant desert cottontail rabbit (sib 1) with a disseminated Staphylococcus aureus infection. (a) Pustules on the lower lip. (b) Front paw showing swelling and inflammation.
During the necropsy of sib 1, abscesses were found in both front paws (Fig. 3a, b), on the chin, and on the lower lip. Multiple small subcutaneous abscesses (Fig. 4), as well as abscesses in the kidneys (Fig. 5a), lungs (Fig. 5b), and the inside of the heart were found. Gram stains of exudate from the abscesses showed many gram-positive cocci in grape-like clusters (Fig. 6). A presumptive diagnosis of S. aureus was made at the CWRF, and this diagnosis was later confirmed by culture at a commercial veterinary diagnostic laboratory. According to the laboratory’s report, after growing “very many” S. aureus bacteria on the preliminary culture, no additional growth was observed. In addition, no growth was detected on an anaerobic culture. However, given the difficulty of collecting anaerobic samples, the presence of anaerobic organisms cannot be ruled out. (The specimen was collected aseptically from deep within the abscess and immediately placed into a BD BBL™ CultureSwab™ Plus collector.) The antibiotic susceptibility test showed susceptibility to all antibiotics tested, including TMP-SMZ and other antibiotics often used in rabbits (Table 1). However, the susceptibility interpretations were mostly of canine origin, as none were available for lagomorphs. The laboratory reported that this pathogen was also susceptible to methicillin.
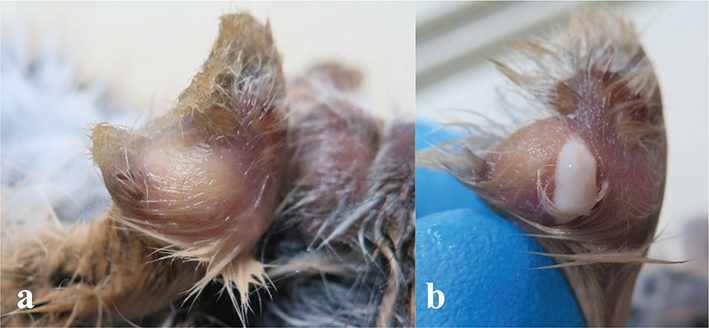
Fig. 3 Necropsy of an infant desert cottontail rabbit (sib 1) with a disseminated Staphylococcus aureus infection, showing large abscesses in the right (a) and left (b) front paws. Slight pressure on the left abscess caused it to rupture, as seen in (b).
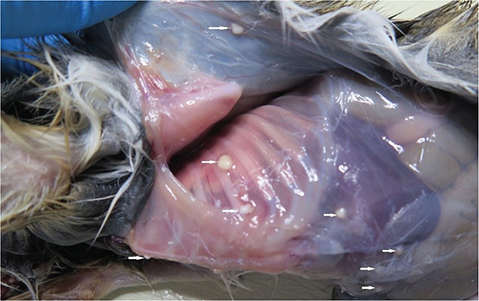
Fig. 4 Necropsy of an infant desert cottontail rabbit (sib 1) with a disseminated Staphylococcus aureus infection, showing many small subcutaneous abscesses.

Fig. 5 Necropsy of an infant desert cottontail rabbit (sib 1) with a disseminated Staphylococcus aureus infection. (a) Abscesses in the kidney. (b) Abscesses in the lungs.

Fig. 6 Gram stain of abscess exudate from an infant desert cottontail rabbit (sib 1) with a disseminated Staphylococcus aureus infection, showing clusters of gram-positive cocci (1000x).
Sib 2 remained apparently healthy, continued to gain weight, and was successfully weaned. However, on day 13, crusty lesions were observed on one corner of the mouth, and one front paw appeared swollen. The rabbit was then prescribed TMP-SMZ, but at higher dose than had been given to sib 1 (TMP-SMZ, 40 mg/kg, PO, BID [Varga Smith 2023c]). The lesions on the mouth and the swollen paw seemed to improve after a few days; however, intermittent raspy breathing and wheezing were observed on day 18. The respiratory signs appeared to subside; however, on day 21, the lesion on the mouth re-appeared, and a large swelling on the neck became apparent (Fig. 7). On day 22, the decision was to euthanize was taken.

Fig. 7 A young desert cottontail rabbit (sib 2) with a disseminated Staphylococcus aureus infection, showing a crusty lesion on the corner of the mouth and a large swelling on the neck.
The necropsy of sib 2 showed a large abscess on the neck and multiple small abscesses and crusty lesions on the chin, neck, and toes (Fig. 8). No abscesses were found within the body cavity; however, the lungs appeared mottled (Fig. 9). A gram stain of exudate from the large neck abscess showed many gram-positive cocci, and S. aureus was again confirmed via culture. The susceptibility test also showed susceptibility to all antibiotics tested, including TMP-SMZ.

Fig. 8 Necropsy of a young desert cottontail rabbit (sib 2) with a disseminated Staphylococcus aureus infection. (a) Several small abscesses and crusty lesions on the chin and neck, as well as a large abscess on the neck. (b) Crusty lesions on the toes.

Fig. 9 Necropsy of a young desert cottontail rabbit (sib 2) with a disseminated Staphylococcus aureus infection, showing mottled lungs.
Discussion
The lesions observed in these two cases were very similar to those described in S. aureus infections by Corpa et al. (2009) in domestic rabbits and by McCoy & Steenbergen (1969) in eastern cottontail rabbits (S. floridanus). While those cases were described as “outbreaks” with high mortality (Corpa et al. 2009) or “epizootics” (McCoy & Steenbergen 1969), the CWRF saw no apparent transmission of the pathogen to other patients at the CWRF facility, except possibly between the two siblings. Since S. aureus is primarily spread through direct contact (Corpa et al. 2009), widespread transmission at the CWRF may have been prevented because patients were housed separately in large plastic tubs, either as individuals or in small groups (mostly litter-based) in the case of younger rabbits. This prevented direct contact between individuals not housed together. Whether the strain of S. aureus in these cases fell into the high-virulence or low-virulence category was not determined. The authors suspect that both infections described here were acquired in the wild, while the kits were nursing, a common route of transmission in domestic rabbits (Corpa et al. 2009). This suspicion is supported by the observation that the lesions first appeared on the mouths and front paws, and by the absence of any visible injuries. However, since initial clinical signs in sib 2 appeared almost a week later than in sib 1, spread between the siblings cannot be ruled out.
Each rabbit was prescribed an antibiotic as soon as clinical signs were observed. However, it is important to note that the initial signs in both rabbits were subtle. In both cases, appetite, weight gain, feces, and activity levels appeared normal until late in the course of the disease. Once clinical signs were noticed in sib 1, the disease progressed quickly, and the rabbit was euthanized three days later due to rapidly declining health. Sib 2 initially appeared to respond to antibiotic treatment; by the time it became clear that the infection was continuing to fester, a large abscess had already developed on the neck. Given that these cases presented during the height of the busy season at the CWRF, it is possible that an earlier diagnosis could have been achieved through more careful observation and heightened awareness among the caretakers. Whether this would have altered the outcomes in these two cases is not known.
Disseminated S. aureus cases have been infrequently diagnosed at the CWRF facility. These have typically been found in adult cottontail rabbits, and the disease was usually too advanced at intake to attempt treatment. The necropsy pictured in Fig. 10a shows an example of an infection possibly originating from mastitis, and Fig. 10b shows an example possibly originating from a large abscess in the axilla. Staphylococcus aureus was confirmed through culture in both adult cases. The sib 1 and sib 2 cases are the first confirmed cases in infant rabbits at the CWRF. However, disseminated abscesses have occasionally been seen on necropsy in other infants; these may have been staph infections, but the pathogen was not identified. All cases at the CWRF appeared isolated and were not suspected of being part of a larger epizootic outbreak.

Fig. 10 Necropsies of adult desert cottontail rabbits with disseminated Staphylococcus aureus infections. (a) Shows mastitis and small abscesses on the chest wall and liver. (b) Shows a large abscess in the axilla and small abscesses on the heart.
The specific pathogen affecting sib 1 and sib 2 was reported to be susceptible to all the antibiotics tested (Table 1), as well as to methicillin. It is important to note, however, that two cases of methicillin-resistant S. aureus (MRSA) were found in eastern cottontail rabbits during a pilot screening study at an Iowa wildlife rehabilitation center (Wardyn et al. 2012). As the potential for zoonotic transmission exists, and both methicillin-susceptible S. aureus as well as MRSA can result in serious diseases in humans (CDC 2019; Cheung et al. 2021), rehabilitators should exercise caution when working with suspected S. aureus cases.
Despite being susceptible to TMP-SMZ, this antibiotic was ineffective at treating the infections in sib 1 and sib 2, and both rabbits required euthanasia. It is possible that sib 1 was diagnosed too late in the course of the disease for any antibiotic to have been effective. In sib 2, however, while TMP-SMZ seemed to have slowed the progression of the disease, it ultimately was inadequate as a treatment, even at the higher dose. One factor which may have played a role is that TMP-SMZ can be inactivated by the presence of pus and other debris in infected tissue (Goldstein & Proctor 2008; Budde & McCluskey 2023; Varga Smith 2023c). Staphylococcus aureus (as well as Pasteurella spp.) infections are common in rabbits and are known to cause purulent infections (Ferreira et al. 2014; Espinosa et al. 2020). In experimental studies, TMP-SMZ has been shown to become ineffective in rabbits in as few as 24 hours after challenge with S. aureus (de Go´rgolas et al. 1995). Different antibiotics may have resulted in better outcomes and should be tried in future cases. Corpa et al. (2009) indicated that tetracycline may afford some benefit in domestic rabbits. Unfortunately, antibiotic choices for cottontail rabbits are limited, partly due to the sensitive nature of rabbits to many antibiotics (Varga Smith 2023c), but also because cottontail rabbits are a hunted species in many states, and as such are considered food animals; many drugs otherwise used in rabbit medicine are thus prohibited or require long withdrawal periods in cottontail rabbits (Schott 2017).
Conclusion
While disseminated S. aureus infections have only been diagnosed in a few individual cases at the CWRF, this pathogen is known to have the potential for epizootics with high morbidity and mortality. Staphylococcus aureus can be spread through direct contact, and therefore proper biosecurity protocols are important. As described in these two cases, initial clinical signs can be subtle, and progression of the disease can be rapid in the absence of antibiotic treatment. Even with antibiotics, treatment is difficult, given both the potential virulence of the pathogen and the limited choices of antibiotics available for treating cottontail rabbits. TMP-SMZ, a frequent first-choice antibiotic used in cottontail rabbit rehabilitation, was ineffective at treating disseminated S. aureus in the cases presented here. Other treatments, probably using different antibiotics, are needed to improve outcomes. Among the myriad pathogens to which cottontail rabbits are susceptible, specific clinical signs may help identify the possible agent and lead to the appropriate treatment. It is hoped that this case report of S. aureus in infant cottontail rabbits will contribute to the rehabilitators’ toolbox for diagnosing and treating their patients.
Acknowledgements
The authors thank all of those who rescue wild animals experiencing crisis and our colleagues in the wildlife rehabilitation community who care for them.
Disclosure statement
The authors report no conflict of interest.
Funding
The authors received no specific funding for this study.
References
| Attili A.-R., Nebbia P., Bellato A., Galosi L., Papeschi C., Rossi G., Linardi M., Fileni E., Cuteri V., Chiesa F. & Robino P. 2020. The effect of age and sampling site on the outcome of Staphylococcus aureus infection in a rabbit (Oryctolagus cuniculus) farm in Italy. Animals 10(5), 774. doi: 10.3390/ani10050774 |
| Barthold S.W., Griffey S.M. & Percy D.H. (eds.) 2016. Rabbit. In Pathology of laboratory rodents and rabbits. 4th ed. Pp. 285–286. Hoboken, NJ: Wiley-Blackwell. doi: 10.1002/9781118924051.ch06 |
| Budde J.A. & McCluskey D.M. 2023. Sulfa-/Trimethoprim. In Plumb’s veterinary drug handbook. 10th ed. P. 1193. Hoboken, NJ: Wiley-Blackwell. |
| CDC. 2019. Deadly Staph infections still threaten the U.S. Accessed on the internet at https://archive.cdc.gov/#/details?url=https://www.cdc.gov/media/releases/2019/p0305-deadly-staph-infections.html on 9 January 2024. |
| Cheung G.Y.C., Bae J.S. & Otto M. 2021. Pathogenicity and virulence of Staphylococcus aureus. Virulence 12(1), 547–569. doi: 10.1080/21505594.2021.1878688 |
| Corpa J., Hermans K. & Haesebrouck F. 2009. Main pathologies associated with Staphylococcus aureus infections in rabbits: a review. World Rabbit Science 17, 115–125. doi: 10.4995/wrs.2009.651 |
| de Go´rgolas M., Aviles P., Verdejo C. & Fernandez Guerrero M.L. 1995. Treatment of experimental endocarditis due to methicillin-susceptible or methicillin-resistant Staphylococcus aureus with trimethoprim-sulfamethoxazole and antibiotics that inhibit cell wall synthesis. Antimicrobial Agents and Chemotherapy 39, 953–957. doi: 10.1128/AAC.39.4.953 |
| Espinosa J., Ferreras M.C., Benavides J., Cuesta N., Pérez C., García Iglesias M.J., García Marín J.F. & Pérez V. 2020. Causes of mortality and disease in rabbits and hares: a retrospective study. Animals 10(1), 158. doi: 10.3390/ani10010158 |
| Ferreira A., Monteiro J.M. & Vieira-Pinto M. 2014. The importance of subcutaneous abscess infection by Pasteurella spp. and Staphylococcus aureus as a cause of meat condemnation in slaughtered commercial rabbits. World Rabbit Science 22(4), 311–317. doi: 10.4995/wrs.2014.2238 |
| Fisher P. & Graham J. 2018. Antimicrobial agents used in rabbits. In J.W. Carpenter (ed.): Exotic animal formulary. 5th ed. Pp. 500–502. St. Louis, MO: Elsevier. |
| Goldstein E.J.C. & Proctor R.A. 2008. Role of folate antagonists in the treatment of methicillin-resistant Staphylococcus aureus infection. Clinical Infectious Diseases 46(4), 584–593. doi: 10.1086/525536 |
| Hermans K., De Herdt P., Devriese L.A., Hendrickx W., Godard C. & Haesebrouck F. 1999. Colonization of rabbits with Staphylococcus aureus in flocks with and without chronic staphylococcosis. Veterinary Microbiology 67(1), 37–46. doi: 10.1016/S0378-1135(99)00028-0 |
| Jangsangthong A., Suriyakhun N., Tunyong W., Kong-Ngoen T., Santajit S., Indrawattana N. & Buranasinsup S. 2022. Occurrence of antimicrobial resistance and antimicrobial resistance genes in methicillin-resistant Staphylococcus aureus isolated from healthy rabbits, Veterinary World 15(11), 2699–2704. doi: 10.14202/vetworld.2022.2699-2704 |
| Markey B.K., Leonard F.C., Archambault M., Cullinane A. & Maguire D. 2013. Staphylococcus species. In Clinical veterinary microbiology, 2nd ed. P. 112. Edinburgh: Elsevier. |
| McCoy R.H. & Steenbergen F. 1969. Staphylococcus epizootic in western Oregon cottontails. Bulletin of the Wildlife Disease Association 5(1), 11. doi: 10.7589/0090-3558-5.1.11 |
| Miller E.A., Goodman M. & Cox S., (eds.) 2017. Sulfamethoxazole (SMZ)/Trimethoprim (TMP). In NWRA Wildlife Formulary. 4th ed. P. 121. St. Cloud, MN: The National Wildlife Rehabilitators Association. |
| Ranzani O.T., Motos A., Chiurazzi C., Ceccato A., Rinaudo M., Bassi G.L., Ferrer M. & Torres A. 2020. Diagnostic accuracy of gram staining when predicting staphylococcal hospital-acquired pneumonia and ventilator-associated pneumonia: a systematic review and meta-analysis. Clinical Microbiology and Infection 26(11), 1456–1463. doi: 10.1016/j.cmi.2020.08.015 |
| Schott R. 2017. Extra-label drug use in wildlife rehabilitation medicine. Wildlife Rehabilitation Bulletin 35(2), 33–36. doi: 10.53607/wrb.v35.20 |
| Spaulding A.R., Satterwhite E.A., Lin Y.C., Chuang-Smith O.N., Frank K.L., Merriman J.A., Schaefers M.M., Yarwood J.M., Peterson M.L. & Schlievert P.M. 2012. Comparison of Staphylococcus aureus strains for ability to cause infective endocarditis and lethal sepsis in rabbits. Frontiers in Cellular and Infection Microbiology 2, 18. doi: 10.3389/fcimb.2012.00018 |
| Stewart G.C. 2022. Staphylococcus. In D.S. McVey et al. (eds.): Veterinary microbiology. 4th ed. Pp. 231–239. Hoboken, NJ: Wiley-Blackwell. doi: 10.1002/9781119650836.ch25 |
| Varga Smith M. 2023a. Infectious diseases of domestic rabbits. In Textbook of rabbit medicine. 3rd ed. Pp. 352–353. Edinburgh: Elsevier. |
| Varga Smith M. 2023b. Abscesses. In Textbook of rabbit medicine. 3rd ed. Pp. 224–225. Edinburgh: Elsevier. |
| Varga Smith M. 2023c. Therapeutics. In Textbook of rabbit medicine. 3rd ed. Pp. 100–137. Edinburgh: Elsevier. |
| Wardyn S., Kauffman L. & Smith T. 2012. Methicillin-resistant Staphylococcus aureus in Central Iowa Wildlife. Journal of Wildlife Diseases 48, 1069–1073. doi: 10.7589/2011-10-295 |