CASE REPORT
Strigid herpesvirus-1 as a differential for chronic keratitis in owls
Sara Childs-Sanford,1,2 Cynthia Hopf-Dennis1,2 & Eric Ledbetter1
1The Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY, USA; 2The Janet L. Swanson Wildlife Hospital, College of Veterinary Medicine, Cornell University, Ithaca, NY, USA
Abstract
An adult free-ranging great horned owl was referred for evaluation of chronic ulcerative keratitis in the left eye that had been treated for several months. In vivo confocal microscopy confirmed that the corneal lesion was an inactive scar, and histopathology and molecular testing of a proliferative palpebral conjunctival lesion in the same eye confirmed infection with strigid herpesvirus- 1. Resolution of the conjunctivitis occurred following the biopsy and the owl was released. Four months later the owl re-presented with presumed rodenticide poisoning, and there was no evidence of recurrence of ocular disease.
BIO
Sara Childs-Sanford an Associate Professor and Section Chief of Wildlife Medicine at Cornell University, and a diplomate of the American College of Zoologial Medicine. She is a clinician at the Janet L. Swanson Wildlife Hospital, where she also teaches veterinary students, trains interns and residents, and performs wildlife health research.
Cynthia Hopf-Dennis is a Clinical Assistant Professor of Wildlife Medicine at Cornell University, and a diplomate of the American College of Zoologial Medicine. She practices and teaches clinical wildlife medicine at the Janet L. Swanson Wildlife Hospital and has a particular interest in avian orthopedics.
Eric Ledbetter is a diplomate of the American College of Veterinary Ophthalmologists and the James Law Professor of Ophthalmology at the Cornell University College of Veterinary Medicine where he serves as the Ophthalmology Section Chief. In addition to research and teaching endeavors, Dr. Ledbetter provides clinical ophthalmology services within Cornell University’s Companion Animal and Equine & Farm Animal Hospitals.
Keywords
Great horned owl; Bubo virginianus; herpesvirus; conjunctivitis
Abbreviations
AST: aminotransferase
CK: creatine kinase
PCR: polymerase chain reaction
StrHV-1: Strigid herpesvirus-1
SWH: Janet L. Swanson Wildlife Hospital
Citation: Wildlife Rehabilitation Bulletin 2023, 41(2), 28–31, http://dx.doi.org/10.53607/wrb.v42.265
Copyright: Wildlife Rehabilitation Bulletin 2023. © 2023 S. Childs-Sanford et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Accepted: 1 November 2023; Published: 15 December 2023
Competing interests and funding: The authors report no conflict of interest.
The authors have no funding sources to report.
Correspondence: Sara Childs-Sanford, The Janet L. Swanson Wildlife Hospital, 131 Swanson Drive, Ithaca, NY 14850 USA. E-mail: sec15@cornell.edu
Case report
In September 2022, an adult male great horned owl was referred to the Janet L. Swanson Wildlife Hospital (SWH) from a licensed wildlife rehabilitator and consulting veterinarian. The owl had originally presented to the rehabilitator approximately six months prior, with advanced lesions in the left eye described as chronic corneal ulceration and bullous keratopathy. The eye was treated with multiple courses of topical antibiotics and antifungal medications. Additionally, the owl had also been assessed by a consulting veterinary ophthalmologist who performed a corneal cytology, which was negative for infectious agents, as well as thermokeratoplasty, which resulted in minimal improvement in the lesion appearance. The owl was referred for additional diagnostics and evaluation to understand the nature of the lesion, assess the overall health of the owl, and determine if eye removal (evisceration) was indicated (Murray et al. 2013).
On physical examination the owl was thin, with a body condition score of 3 out of 9. There was a large thick irregular white plaque over the dorsolateral cornea, as well as a mass of hyperemic proliferative conjunctiva covering about 50% of the inner surface of the lower left eyelid (Figure 1). Initial diagnostic testing included a complete blood count, chemistry panel, radiographs, and corneal swab cytology and culture. The complete blood count showed hemoparasitism with moderate levels of Hemoproteus spp. and Plasmodium spp., as well as few Leukocytozoon spp. The chemistry panel showed moderate elevation of aspartate aminotransferase (AST) and creatine kinase (CK). Cytology of the cornea showed heterophilic inflammation and few extracellular gram-positive cocci. Bacterial culture of the corneal swab grew few Enteroccus casseliflavus isolated from the broth, and fungal culture was negative. Fecal parasitology showed a moderate level of Capillaria spp. nematode eggs. The owl was started on treatment for hemoparasites and gastrointestinal parasites, as well as oral meloxicam and topical ciprofloxacin drops in the left eye.
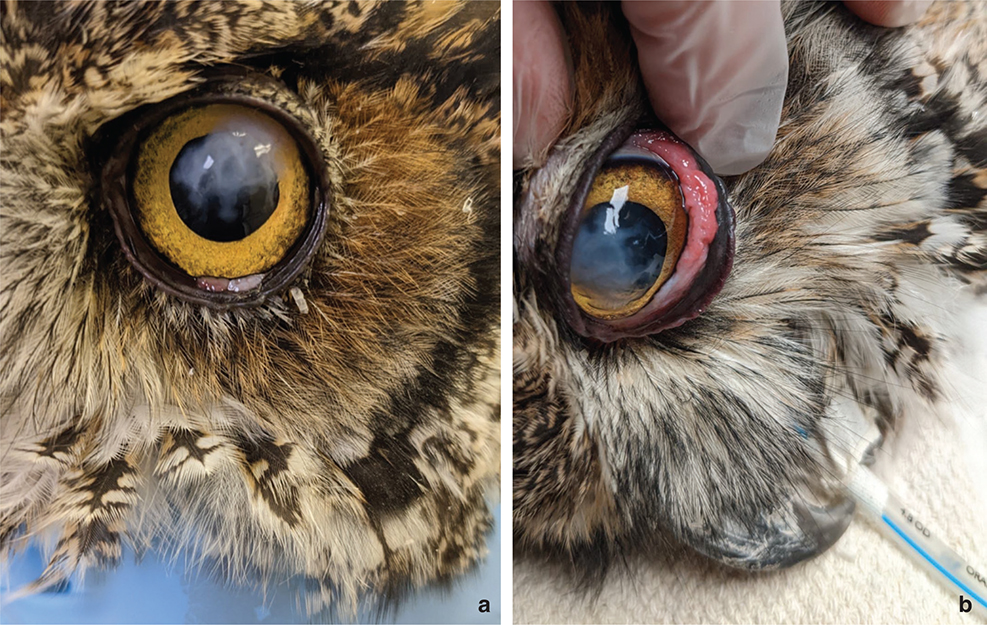
Fig. 1 (a and b) A dorsolateral white corneal opacity with irregular margins is present in the left eye of this great horned owl (a). A small light pink mass of conjunctiva can be seen at the margin of the lower eyelid. When the eyelid is retracted, an irregular hyperemic diffuse mass of conjunctival tissue is present (b).
Evaluation of the eyes was performed by the Ophthalmology Service of the Cornell University Hospital for Animals including slit lamp biomicroscopy, and indirect and direct ophthalmoscopy. Ulceration of the left cornea was not present, confirmed by a negative fluorescein stain in both eyes. The right eye had two small linear areas of fibrosis in the cornea (scarring) and a small peripheral retinal detachment located dorsally. The left eye had a large superficial white corneal plaque associated with focal corneal edema and peripheral neovascularization. The anterior chamber of both eyes was normal. Due to a suspicion of mycotic keratitis in the left eye, in vivo confocal microscopy was performed. No active inflammation or infection was found, and the corneal lesion was confirmed to be inactive scar tissue.
A surgical biopsy was taken of the proliferative palpebra conjunctival lesion on the inner surface of the lower left eyelid. Histopathology showed chronic, moderate, locally extensive proliferative conjunctivitis. In addition, intranuclear inclusion structures consistent with a herpesvirus, were seen in a large number of the cells. Based on these findings, a diagnosis of chronic conjunctivitis and keratitis secondary to strigid herpesvirus-1 (StrHV-1) was made. Subsequent DNA extraction from the formalin-fixed conjunctival mass was processed for pan-herpesvirus PCR and gene sequencing confirmed this as the diagnosis. The owl continued treatment with topical ciprofloxacin and oral meloxicam for one more week following the biopsy, to prevent infection and reduce inflammation, and was discharged back to the care of the wildlife rehabilitator. It continued to do well and was released to the wild approximately three weeks later, following successful live prey testing. Approximately four months after release the owl was observed to fall out of a tree and was returned to the SWH, where it was diagnosed with anticoagulant rodenticide poisoning. The corneal scar was observed to be unchanged, and there were no proliferative conjunctival lesions or evidence of conjunctivitis. The owl responded well to treatment and was eventually released in a different location.
Discussion
Ocular lesions are common in owls that present to wildlife veterinarians and rehabilitators. Most often these are traumatic in origin, with owl eyes being predisposed to injury due to their large size and unique anatomy that leave them less protected than in many other animal species (Davidson 1997; Vallone & Kern 2021). Complete examination of the eye, including both the anterior and posterior segments, is vital to determining the extent of ocular damage in any bird of prey that presents to a wildlife rehabilitation or hospital setting, since visual compromise may impact hunting ability and survival success post-release (Labelle et al. 2012).
Infectious causes of ocular lesions are less common than traumatic injury but do occur, and most often this is in the form of secondary infection following traumatic injury to the ocular tissues. Corneal erosion and ulceration secondary to trauma is the most commonly encountered cause of corneal lesions in owls. In a survey of traumatic ocular lesions in nocturnal raptors, corneal ulceration was the cause of 57% of the ocular lesions observed in Eurasian scops owls (Otus scops) (Seruca et al. 2012). In addition to bacterial agents, secondary infection with fungal organisms can occur. Fungal infection of the cornea has been reported in many species of birds, including psittacines, poultry, waterfowl, cormorants and gulls, with numerous genera of causative fungal agents isolated (Willis & Wilkie 1999; Griggs 2019). A recent case series of avian mycotic keratitis included two owls (Bubo scandiacus and Strix varia) (Lucyshyn et al. 2023). Corneal lesions in these cases were described as including corneal opacity and ulceration, similar in appearance to the lesion in the great horned owl in this case, in which mycotic keratitis was considered a top differential. Treatment of fungal keratitis often takes weeks to months and the prognosis for resolution is guarded to poor, often requiring ocular evisceration or enucleation. Eye removal can be curative in some cases, with an owl that has a normal (no lesions) remaining eye being releasable following ocular evisceration (and successful rehabilitation and prey testing) and nonreleasable following enucleation.
In vivo confocal microscopy is a highly effective and noninvasive diagnostic tool that can determine the presence and extent of corneal infiltration by fungal hyphae, as well as aid in treatment monitoring and prognosis, and its use for this has been reported in owls (Lucyshyn et al. 2023). In this great horned owl case, in vivo confocal microscopy was important in ruling out mycotic keratitis. Fungal culture of the lesion is not as sensitive of a test and may take several weeks to be finalized. In contrast, in vivo confocal microscopy provides an immediate result. Its use in this case resulted in the timely diagnosis of a corneal scar, allowing us to more accurately determine diagnostic and treatment plans and transfer the owl back to the rehabilitator much sooner.
Primary infectious causes of ocular lesions in owls are rare. StrHV-1 has been previously reported in a captive great horned owl in California (Gleeson et al. 2019). Clinical signs were very similar to those in this case, with chronic keratitis accompanied by proliferative conjunctivitis on the inside of the lower eyelid of the same eye. Also similarly, biopsy of the conjunctivitis revealed characteristic intranuclear herpesvirus inclusions. Phylogenetic analysis of the virus in this previous case identified a new, genetically distinct herpesvirus in the subfamily Alphaherpesvirinae. This previous case eventually resolved with a combination of diamond burr debridement of the corneal lesion and medical therapy that included antiviral drugs, although an addendum to the report describes a second owl treated with this regimen that did not respond and was euthanized. Antiviral drugs were not used in the owl in this case to achieve resolution due to unknown efficacy of the drugs in birds and for this specific virus. Research on treatment options and their efficacy would be beneficial in cases of StrHV-1 conjunctivitis in owls, and the pathogenesis of the disease and potential primary role of the conjunctival location and lesion causing corneal disease should be considered.
It was proposed in Gleeson et al. (2019) that the proliferative conjunctivitis on the lower eyelid was the primary lesion, resulting in mechanical trauma to the cornea. This also seemed to be true in this case, where the corneal scar was created over time and appeared to correlate perfectly in location to where the proliferative conjunctival lesion on the inner lower lid would contact the ocular surface when the eyelid was closed. Repeated corneal trauma over time may have caused corneal ulceration, which was successfully treated by the referring veterinarian and ophthalmologist and resulted in a residual scar that remained static as the proliferative conjunctivitis completed healing and for months after release. Since StrHV-1 is a herpesvirus, lifelong infection can be assumed, with a risk of latency being interrupted by periods of disease activation. In this case, the owl was exposed to anticoagulant rodenticide after release, and despite this illness followed by several weeks of hospitalization and rehabilitation, no recurrence of ocular disease was noticed.
Chronic ocular disease and severe lesions in raptors can take many weeks to even months of treatment, with topical ophthalmic medications requiring application at least twice daily in order to be effective. This can be stressful for patients and general health and well-being and quality of life should be regularly assessed. This case demonstrates the importance of veterinary evaluation of chronic ocular lesions in raptors, including owls. The completion of advanced diagnostic testing to confirm an accurate diagnosis so correct treatment and prognosis can be determined in a timely fashion, can help to minimize prolonged hospitalization. StrHV-1 should be considered a differential for chronic corneal lesions in owls, and the conjunctiva should be carefully evaluated for proliferative lesions. Histopathology of the conjunctival lesion was instrumental in the diagnosis in this case, and in vivo confocal microscopy offered a rapid method of ruling out mycotic or other infectious keratitis and allowed cessation of topical treatment of the corneal lesion. Further research is needed to understand the epidemiology of this StrHV-1 in owls including host distribution, modes of transmission, health impact, and treatment.
References
| Davidson M. 1997. Ocular consequences of trauma in raptors. Seminars in Avian and Exotic Pet Medicine 6, 121–130, doi: 10.1016/S1055-937X(97)80019-9. |
| Gleeson M.D., Moore B.A., Edwards S.G., Stevens S., Childress A.L., Wellehan J.F.X., Robertson J., Murphy C.J., Hawkins M.G. & Paul-Murphy J. 2019. A novel herpesvirus associated with chronic superficial keratitis and proliferative conjunctivitis in a great horned owl (Bubo virginianus). Veterinary Ophthalmology 22(1), 67–75, doi: 10.1111/vop.12570. |
| Griggs A. 2019. Ocular surface disease in birds. Veterinary Clinics of North America: Exotic Animal Practice 22(1), 53–68, doi: 10.1016/j.cvex.2018.08.005. |
| Labelle A.L., Whittington J.K., Breaux C.B., Labelle P., Mitchell M.A., Zarfoss M.K., Schmidt S.A. & Hamor R.E. 2012. Clinical utility of a complete diagnostic protocol for the ocular evaluation of free-living raptors. Veterinary Ophthalmology 15(1), 5–18, doi: 10.1111/j.1463-5224.2011.00899.x. |
| Lucyshyn D.R., Childs-Sanford S.E., Choi E. & Ledbetter E.C. 2023. In vivo confocal microscopy for characterization of mycotic keratitis in owls (Bubo scandiacus, Strix varia) and a woodcock (Scolopax minor): 3 cases. Journal of Zoo and Wildlife Medicine 54(1), 202–210, doi: 10.1638/2022-0071. |
| Murray M., Pizzirani S. & Tseng F. 2013. A technique for evisceration as an alternative to enucleation in birds of prey: 19 cases. Journal of Avian Medicine and Surgery 27(2), 120–127, doi: 10.1647/2012-007. |
| Seruca C., Molina-Lopez R., Pena T. & Leiva M. 2012. Ocular consequences of blunt trauma in two species of nocturnal raptors (Athene noctua and Otus scops). Veterinary Ophthalmology 15(4), 236–244, doi: 10.1111/j.1463-5224.2011.00976.x. |
| Vallone L.V. & Kern T.J. 2021. Avian ophthalmology. In K.N. Gelatt (ed.): Veterinary ophthalmology. Pp. 2055–2084. Sixth edition, volume 2. Hoboken, NJ: John Wiley & Sons, Inc. |
| Willis A.M. & Wilkie D.A. 1999. Avian ophthalmology, part 2: review of ophthalmic diseases. Journal of Avian Medicine and Surgery 13(4), 245–251. |