ARTICLE
Prevalence of HPAI in passerines, woodpeckers and doves presented to the Center for Wild Bird Rehabilitation during the 2022 HPAI H5N1 epizootic
Bren Lundborg & Grae O’Toole
Center for Wild Bird Rehabilitation, Vermont Institute of Natural Science, Quechee, Vermont
Abstract
The Highly Pathogenic Avian Influenza (HPAI) H5N1 clade 2.3.4.4b epizootic affecting North America beginning in late 2021 has affected wild and domestic birds to an unprecedented scale. Mortalities have been documented mainly in raptors, aquatic birds (shorebirds, waterfowl), gulls and other scavenging birds, with rare reports of other species being affected. Passerines, woodpeckers, and doves are not thought to contribute significantly to the transmission of avian influenza, and thus are not regularly tested. Despite this, mortalities and even the possibility of asymptomatic carrier states in songbirds have been documented with HPAI H5N1 strains. This study focused on sampling and testing of species not typically included in HPAI surveillance, to help assess prevalence, symptoms of infection if detected and the possibility of asymptomatic carriers. Throughout two periods in 2022, 164 individuals of 38 species were sampled from Vermont and New Hampshire. None of these birds tested positive for any strain of avian influenza virus. While the scale of this study is small, it highlights the need for testing species that are not a part of regular HPAI surveillance, as well as the role rehabilitation centers may play in that goal.
BIO
Bren Lundborg is the Lead Wildlife Keeper at the Vermont Institute of Natural Science (VINS). He has been working in CWBR, the rehabilitation department of VINS since 2017 and leads research projects in the rehabilitation department.
Grae O’Toole is the Director at the Vermont Institute of Natural Science (VINS). She has been working in CWBR, the rehabilitation department at VINS since 2017. She is responsible for the overall management and curatorial duties of all live animals at VINS.
Keywords
Influenza; HPAI; H5N1; passerines; disease
Abbreviations
AIV: avian influenza virus
CWBR: Center for Wild Bird Rehabilitation
HPAI: highly pathogenic avian influenza
Citation: Wildlife Rehabilitation Bulletin 2023, 41(2), 44–48, http://dx.doi.org/10.53607/wrb.v41.264
Copyright: Wildlife Rehabilitation Bulletin 2023. © 2023 B. Lundborg et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Accepted: 1 November 2023; Published: 15 December 2023
Competing interests and funding: The authors report no conflict of interest.
The authors would like to thank Jim Evans as well as the Davis Foundation for supporting this work.
Correspondence: Bren Lundborg, Vermont Institute of Natural Science, E-mail: blundborg@vinsweb.org. 802-359-5001 x216
Introduction
Highly pathogenic avian influenza (HPAI) is an important emerging disease of wild birds and is also a major concern for domestic and captive bird species (Center for Food Security and Public Health [CFSPH], 2016; Caliendo et al. 2022). The ongoing 2021–23 H5N1 epizootic in North America is the largest HPAI outbreak on the continent to date, with an unprecedented impact on wild birds (Harvey et al. 2023). It is generally thought that passerines play a limited role in the spread of avian influenza viruses (AIVs), and given their small size, detections of sick or dead passerines are less likely to occur than raptors or waterfowl. Although uncommon with the exception of large corvids, mortalities have been detected not only in the current epizootic in 14 passerine species, mainly in corvid and grackle species but also in an American robin (Turdus migratorius), dark-eyed junco (Junco hyemalis), tree swallow (Tachycineta bicolor) and a house sparrow (Passer domesticus) (APHIS 2022; CFIA 2023). Criteria for testing of passerines in Vermont requires five or more mortalities of any species within the same visual area, so sick individuals are not likely to be tested (Vermont Fish and Wildlife Department [VTFW] 2022). Additionally, songbirds are not a high priority surveillance target for influenza monitoring, with the focus being on waterfowl since they are known to be the major reservoir of AIVs (APHIS 2017; APHIS 2022).
The current HPAI outbreak is a unique opportunity to study exposure in wild passerines. HPAI outbreaks are historically rare in North America, with only seven prior outbreaks recorded in the United States (APHIS 2016). This outbreak has affected a much higher number of species than the 2014–15 outbreak in which positive cases were found in around 15 wild species, with 98 total cases in wild birds (APHIS 2016; APHIS 2017). Thus far in the current epizootic, the current H5N1 clade 2.3.4.4b has been detected in over 150 species, and over 9750 cases have been reported at the time of this writing (13 October 2023) between the United States and Canada (APHIS 2023b; CFIA 2023).
This study sampled songbirds, woodpeckers and doves from Vermont and New Hampshire during periods of high prevalence of HPAI. The goal was to focus on species not targeted by typical HPAI surveillance protocols to learn more about the prevalence of the HPAI H5N1 strain among these species. Any positive cases detected could help to inform rehabilitators and wildlife agencies about symptoms, species affected and possible asymptomatic carrier states.
Materials and methods
Sampling focused on passerines, woodpeckers and doves presented for care at CWBR. Birds were identified by experienced rehabilitation staff, and age was designated at intake as nestling, fledgling, hatch year, after hatch year, or unknown. The location where the bird was found was collected at intake whenever possible. Efforts were made to sample as many individuals as possible during the sampling periods, however birds were not sampled if they were judged too unstable for extra handling. Rarely, birds were sampled post-mortem when they died during the first 48 h of care and were still isolated from other patients. When groups of nestling birds were presented, only one individual from the group was tested, with the assumption that the highly transmissible virus would infect all individuals in a nest if present. Common ravens (Corvus corax) and American crows (Corvus brachyrhynchos) were excluded from the study due to their relatively unique ecology compared with other Vermont passerines, and regularly documented mortalities due to HPAI (APHIS 2022).
Samples were collected during the periods between 9 May 2022 through 11 July 2022 and 7 October 2022 through 4 December 2022. Although positive cases had been reported through the spring, testing began in early May since prior to that the center was not accepting patients while biosecurity was updated. Due to limitations in funding, sampling was paused during the summer so that it could be focused on spring and fall migration, and when positive cases (reported by APHIS) appeared to be rising in the fall. An oropharyngeal swab was collected within 48 h of intake and while the bird was being kept isolated from other patients. Swabs were placed in a sterile 6 ML tube with five drops of Lactated Ringer’s Solution according to options for sample submission to the Cornell Animal Health Diagnostic Center (AHDC) and immediately stored at −18 °C (0 °F) until testing. Samples were shipped in a cooler with dry ice to the AHDC and were tested using a real-time PCR assay.
Results
Between the two sampling periods, 164 samples from 38 species of birds were collected. Samples were collected from 148 songbirds, 10 woodpeckers and six doves. No birds tested positive for any strain of AIV, however most species were poorly represented. A summary of species and ages sampled can be seen in Table 1.
Samples were collected from most counties in Vermont, as well as two counties from New Hampshire. The number of samples per county was highly variable, with more samples coming from counties that CWBR receives more birds from (Figure 1).
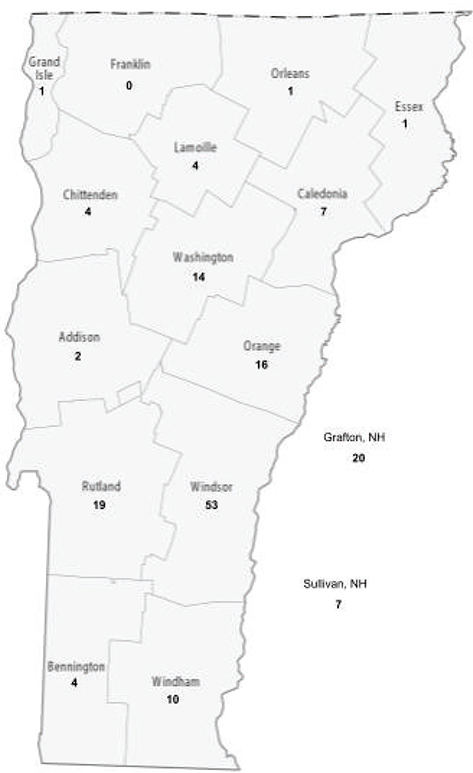
Fig. 1 Number of samples by county (Original map from GIS Geography 2022).
Discussion
While no positive cases of HPAI were detected, the small sample size provides only minimal evidence regarding the risk of HPAI in passerines at rehabilitation centers or elsewhere. There were several limitations to the study. Firstly, the sample size was relatively small, and many samples were from nestling or fledgling birds that were unlikely to be exposed to AIV’s in the environment. The sampling was also from a relatively small geographic area, particularly focused on the regions of Vermont and New Hampshire near Quechee, Vermont where CWBR is located. Another factor may be that most of the species sampled are also highly susceptible to HPAI as has been demonstrated in some passerines, and did not survive long enough to be found and brought to a rehabilitation facility. As has been previously mentioned, sick passerines are less likely to be found than raptors or other large species, so any positive cases are less likely to be detected. A single deceased passerine is much less likely to draw attention than a raptor; CWBR regularly receives calls about deceased raptors but only rarely about deceased passerines.
Despite this, a study by Fuller et al. (2010) found that Passeriformes had the third highest rate of positive influenza samples (0.89%) out of 11 orders, lower only than Anseriformes and Charadriiformes. Influenza viruses were detected in 22 species of passerines, mainly thrushes, sparrows, flycatchers, and warbler species, but the strain of virus was not reported. Some of these species, such as American robins, are regularly found around humans and poultry and are frequently presented to rehabilitation centers. American robins have been the most commonly admitted species for the past five years at CWBR at the Vermont Institute of Natural Science (VINS), with over 100 individuals received between 2020 and 2021 (CWBR unpublished data). Since Fuller et al. reported a 3.76 percent prevalence of AIV’s in American Robins (n = 133) versus 3.99% across all species of Anseriformes, there is potential for songbird species such as this to be a source of significant risk during the current HPAI epizootic.
Although reported HPAI cases in songbirds have been rare, Root et al. (2018) showed that American robins are capable of replicating and shedding H5N2 and H5N8 strains of HPAI without showing clinical symptoms. Fujimoto et al. (2010, 2015) have shown that some passerines are highly susceptible to H5N1 strains, with 100 percent mortality in experimentally infected great reed warblers (Acrocephalus arundinaceous), common reed buntings (Emberiza schoeniclus), and brown eared bulbuls (Hypsipetes amaurotis) but that pale thrushes (Turdus pallidus) may survive infections while shedding virus. An asymptomatic carrier state may be devastating to a rehabilitation facility since an infected bird would pass through a screening process that does not involve testing. Such a carrier state would also suggest a possible mode of movement of AIVs since many passerines are migratory.
While this study is limited in its scope, it illustrates the important role rehabilitation facilities could continue to play in learning more about HPAI in rarely tested species such as passerines, as well as some limitations. Rehabilitation facilities have already played a significant role in monitoring cases in the current epizootic, as well as working hard to maintain the necessary biosecurity (Kalnins & Cox 2023). Facilities cooperating with state and federal agencies could be a useful option for testing large numbers of birds with minimal sampling effort (similar to hunter harvested waterfowl). Such an effort is already underway to study SARS-CoV-2 in wildlife rehabilitation centers (APHIS 2023a). Since many of the species accepted at CWBR (and likely many other centers) are ones that are frequently in close proximity to humans, passerines at rehabilitation centers may be a logical population to sample in the context of a One Health approach. Additionally, the finders have often handled the birds (sometimes for days) before they contact a rehabilitation facility, so human exposure is common in this population.
Perhaps the greatest challenge to further sampling of passerines is funding, both for rehabilitation centers and government agencies. While funding for non-profit wildlife rehabilitation centers such as VINS is often limited, they may have opportunities for funding such work not available elsewhere. For example, this work was funded in part by a private donation. An additional complication is the varied response to the outbreak and positive cases between states. At the beginning of the current outbreak there was significant uncertainty in how different states would respond to a positive case (e.g., culling or quarantine of other animals on site), and such uncertainty would make cooperation between rehabilitation facilities and government agencies challenging.
Overall, this study is a small step toward a better understanding of HPAI prevalence in passerines, and an example of how rehabilitation centers may contribute to this work. The experimental infections referenced in this article as well as reported cases in the current outbreak are a reminder of the importance of biosecurity practices at wildlife rehabilitation facilities, particularly since we know little about HPAI in passerines. Given the growing global incidence of HPAI and its risks to wildlife, poultry, and people, further testing of all wild bird species is needed to understand this rapidly evolving disease.
Conclusion
Passerines, woodpeckers, and doves are regularly accepted to rehabilitation facilities and frequently have close contact with human environments. In light of the massive impact the current H5N1 epizootic has had on wild birds, it is critical to understand how these species are affected by this strain of AIV. While this study had a limited number of samples, it shows the role rehabilitation centers may continue to play in the important goal of better understanding AIVs in all bird species. Work such as this, as well as further testing, is important for biosecurity and risk assessment not only for wildlife rehabilitators but also for agencies working with wild birds, as well as both commercial and private poultry operations.
Acknowledgments
The authors would like to thank Maverick Correia, Falon Cote, and Victoria Stevenson for their help in collecting samples. They would also like to acknowledge the volunteers and finders who made the rescue, transport, and sampling of these birds possible, as well as the organizational support of VINS for making this work possible.
References
| Caliendo V., Leijten L., van de Bildt M.W.G., Fouchier R.A.M., Rijks J.M. & Kuiken T. 2022. Pathology and virology of natural highly pathogenic avian influenza H5N8 infection in wild Common buzzards (Buteo buteo). Scientific Reports 12, 920, doi: 10.1038/s41598-022-04896-7. |
| Canadian Food Inspection Agency (CFIA). 2023. Highly pathogenic avian influenza—wildlife. Last modified March 11, 2023. Accessed on the internet at https://cfia-ncr.maps.arcgis.com/apps/dashboards/89c779e98cdf492c899df23e1c38fdbc on 11 March 2023. |
| Center for Food Security and Public Health (CFSPH). 2016. Highly pathogenic avian influenza fact sheet. Last modified May 2022. Accessed on the internet at https://www.cfsph.iastate.edu/diseaseinfo/disease/?disease=avian-influenza#:~:text=Avian%20influenza%20viruses%20are%20highly,poultry%20are%20also%20readily%20infected on 5 February 2023. |
| Fujimoto Y., Ito H., Shinya K., Yamaguchi T., Usui T., Murase T., Ozaki H., Ono E., Takakuwa H., Otsuki K. & Ito T. 2010. Susceptibility of two species of wild terrestrial birds to infection with a highly pathogenic avian influenza virus of H5N1 subtype. Avian Pathology 39(2), 95–98, doi: 10.1080/03079451003599268. |
| Fujimoto Y., Usui T., Ito H., Ono E. & Ito T. 2015. Susceptibility of wild passerines to subtype H5N1 highly pathogenic avian influenza viruses. Avian Pathology 44(4), 243–247, doi: 10.1080/03079457.2015.1043235. |
| Fuller T.L., Saatchi S.S., Curd E.E., Toffelmeier E., Thomassen H.A., Buermann W., DeSante D.F., Nott M.P., Saracco J.F., Ralph C.J., Alexander J.D., Pollinger J.P. & Smith T.B. 2010. Mapping the risk of avian influenza in wild birds in the US. BMC Infectious Diseases 10, 187, doi: 10.1186/1471-2334-10-187. |
| GIS Geography. 2022. Vermont County Map. Last modified May 25, 2022. Accessed on the internet at https://gisgeography.com/vermont-county-map/ on 5 February 2023. |
| Harvey J.A., Mullinax J.M., Runge M.C. & Prosser D.J. 2023. The changing dynamics of highly pathogenic avian influenza H5N1: next steps for management and science in North America. Biological Conservation 282, 110041, doi: 10.1016/j.biocon.2023.110041. |
| Kalnins G. & Cox S. 2023. On the front lines of wildlife disease. The Wildlife Professional 17(5), 50–52. |
| Root J.J., Bosco-Lauth A.M., Marlenee N.L. & Bowen R.A. 2018. Viral shedding of clade 2.3.4.4 H5 highly pathogenic avian influenza A viruses by American robins. Transboundary Emerging Diseases 65(6), 1823–1827, doi: 10.1111/tbed.12959. |
| USDA Animal and Plant Health Inspection Service (APHIS). 2016. Final report for the 2014–2015 outbreak of Highly Pathogenic Avian Influenza (HPAI) in the United States. Last modified August 11, 2016. Accessed 5 February 2023. https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai/2015-hpai-final-report.pdf |
| USDA Animal and Plant Health Inspection Service (APHIS). 2017. Surveillance methods for detecting avian influenza in wild birds in the U.S. Last modified 2017. Accessed on the internet at https://www.aphis.usda.gov/animal_health/downloads/animal_diseases/ai/surv-methods-avian-influenza-wild-birds.pdf on 5 February 2023. |
| USDA Animal and Plant Health Inspection Service (APHIS). 2022. Implementation plan for avian influenza surveillance in waterfowl in the United States. Last modified 2022. Accessed on the internet at https://www.aphis.usda.gov/animal_health/downloads/animal_diseases/ai/2022-23-wild-bird-ai-surveillance-implementation-plan.pdf on 19 February 2023. |
| USDA Animal and Plant Health Inspection Service (APHIS). 2023a. Investigating SARS-CoV-2 in wildlife rehabilitation facilities. Accessed on the internet at https://cdn.ymaws.com/www.nwrawildlife.org/resource/resmgr/website_2023/usda_covid_research_flyer-20.pdf on 13 October 2023. |
| USDA Animal and Plant Health Inspection Service (APHIS). 2023b. 2022 detections of highly pathogenic avian influenza in wild birds. Last modified October 10, 2023. Accessed on the internet at https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza/hpai-2022/2022-hpai-wild-birds on 13 October 2023. |
| Vermont Fish and Wildlife Department (VTFW). 2022. Avian influenza wildlife health bulletin. Last modified December 27, 2022. Accessed on the internet at https://vtfishandwildlife.com/learn-more/living-with-wildlife/wildlife-diseases/avian-influenza-wildlife-health-bulletin#:~:text=Reporting%20Possible%20Cases%20of%20Wildlife,black%20ducks%2C%20and%20turkey%20vultures on 19 February 2023. |