ARTICLE
Pilot study on the use of soft release for translocation of rehabilitated eastern box turtles (Terrapene carolina carolina)
Jacob E. Alexander, Debbie Sykes & Amanda M.B. Healan
Nashville Wildlife Conservation Center, Nashville, TN, USA
Abstract
Successfully rehabilitated eastern box turtles (EBTs, Terrapene carolina carolina) originating from destroyed or degraded habitats are prime candidates for translocation. However, physiological and behavioral characteristics of chelonians often lead to acclimation difficulties and limited survival post-release. Soft release is a translocation technique whereby an animal is held in a pen on location and allowed to acclimate to its new habitat area for a predetermined duration prior to release. This pilot study explored the viability of small-scale, soft release penning to aid wildlife rehabilitators in the translocation of rehabilitated EBTs. We hypothesized that soft release penning is a practical strategy to help rehabilitated EBTs maintain health and appropriately sized territory in new environments. We built and deployed two soft release pens that were used to relocate three rehabilitated EBTs with varying success to an urban greenspace in Nashville, Tennessee. Our results suggest that soft release penning provides a unique opportunity to observe health and behavior in a controlled environment during the initial period of acclimation, and this early intervention period may help rehabilitators facilitate successful translocation. Further research is recommended to evaluate the direct impacts of soft release pens on body condition, dispersal distance, and home range size of translocated EBTs.
BIO
Jacob E. Alexander is Research Coordinator at NWCC and a Fisheries and Wildlife Sciences student at Oregon State University.
Debbie Sykes, CWR is Executive Director and Founder of NWCC. She has been an NWRA Symposium presenter since 2016, sharing her expertise in animal husbandry, rehabilitation techniques, and wildlife education.
Amanda M.B. Healan, PhD is Board President of NWCC and a science communications consultant. She was also a 2022 NWRA Symposium presenter, leading a workshop on wildlife education, and sharing the present research as well as her grant writing expertise.
Keywords
Wildlife rehabilitation; relocation; acclimation; soft-release; herpetology; eastern box turtle
Abbreviations
NWCC: Nashville Wildlife Conservation Center
BCI: Body Condition Index
EBT: eastern box turtle
Citation: Wildlife Rehabilitation Bulletin 2023, 41(2), 36–43, http://dx.doi.org/10.53607/wrb.v41.259
Copyright: Wildlife Rehabilitation Bulletin 2023. © 2023 J.E. Alexander et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Accepted: 9 October 2023; Published: 15 December 2023
Competing interests and funding: The authors report no conflicts of interest.
NWCC received a grant award from the National Wildlife Rehabilitators Association to partially fund this work.
Correspondence: Debbie Sykes, CWR. E-mail: debbie@nashvillewildlifeconservation.org
Introduction
Eastern box turtles (Terrapene carolina carolina; EBTs) are listed as vulnerable to extinction by the International Union for Conservation of Nature (IUCN), and EBT populations are currently in decline across their native range (van Dijk 2011). Habitat destruction is often cited as a primary contributor to worldwide reptilian population declines (Gibbons et al. 2000), and factors associated with urbanization such as pollution and motor vehicle strikes are primary drivers of high EBT mortality (Brown & Sleeman 2002). Wildlife rehabilitators (rehabilitators) are in a unique position to support the conservation of this imperiled species through the rehabilitation and release of individual box turtles.
Translocation, or the intentional movement of animals from one habitat area to another (Dodd & Seigel 1991), is a conservation management strategy that has been field tested in chelonians (McKee et al. 2021) and may provide rehabilitators with additional release opportunities. Some EBTs are admitted to rehabilitation centers without an adequate description of their native home range, and others may originate from home ranges that are severely degraded or destroyed by the development of urban or suburban infrastructure. Translocation may be a viable option for reintroducing these EBTs to the wild, and relocated EBTs may help reinforce existing populations diminished by urbanization. Many chelonians have strong site fidelity (McKee et al. 2021). Past attempts to relocate box turtles to unfamiliar areas with suitable habitats have seen mixed results (Hester et al. 2008; Henriquez et al. 2017; Poor et al. 2020). Many EBTs disperse toward their home ranges (Cook 2004) and there is some evidence that box turtles face increased mortality and expanded range size after translocation. Dispersal away from release sites or an oversized home range may increase stress due to higher energy use when looking for resources. In addition, this increases post-release opportunities for conflict with humans, roadways or other obstacles that may result in reinjury or even death (Hester et al. 2008). For this reason, novel release and post-release monitoring techniques are needed to assist rehabilitators with EBT translocation, particularly for otherwise healthy rehabilitated EBTs originating from unsuitable or destroyed habitat areas.
One such strategy is soft release, a translocation technique whereby an animal is acclimated for a short time to the novel habitat area prior to release, generally within the confines of a soft release pen. In a recent review, Resende et al. (2021) showed that, when compared to hard release methods, soft release improved outcomes of translocation for terrestrial animals, especially among reptiles. Soft release penning may benefit translocated chelonians by decreasing dispersal distances from the release site (Attum & Cutshall 2015), reducing home range sizes (Tuberville et al. 2005; Frederick 2009), and limiting predation during the acclimation period (Tetzlaff et al. 2019).
Some conservationists may view soft release as an expensive and time-consuming strategy for translocation, but rehabilitators are in a unique position to facilitate small-scale soft release penning through existing partnerships with local landowners and stakeholders. Many rehabilitators may already have the knowledge, staff, and resources required to prepare soft release enclosures and effectively monitor and care for the EBTs during and after the acclimation period, making it a practical option. This pilot study sought to determine the feasibility of small-scale soft release penning as a tool to support translocation of rehabilitated EBTs by wildlife rehabilitators. We hypothesized that soft release penning is a practical strategy to help rehabilitated EBTs maintain health and appropriately sized territory in new environments when compared to sympatric wild EBTs.
Materials & methods
Study area
This study was approved by Metropolitan Nashville Parks and Recreation and the Tennessee Wildlife Resources Agency. The study area, a more than 935-acre mixed use natural area in Davidson County, Tennessee, is made up of mixed riparian, wetland, and deciduous forest habitat and is known to support an active population of EBTs. Two independent soft release pen sites approximately 3 km apart were chosen: Site A and Site B. Sites were selected away from trails in areas with mixed ground and canopy cover. The first pen was installed on 29 May 2021 and the last EBT observation in the field for this study occurred on 28 August 2022.
Rehabilitated turtles
Three EBTs admitted with injury to Nashville Wildlife Conservation Center (NWCC) were selected for the study following their rehabilitation: Rehab M1, Rehab M2, and Rehab F. All three EBTs originated from severely degraded habitats at least 20 kilometers outside of the study area. The relevant landowners had no interest in the release of rehabilitated wildlife on their properties. Further, Rehab M2 was deemed unfit for release without regular monitoring due to potentially restricted mobility.
Rehab M1 was an adult male struck by a motor vehicle and admitted to NWCC on 25 May 2020 from a location approximately 75 kilometers southwest of the study area. The left eye was missing and carapace fractured, exposing the left lung. Rehab M1 received standard wound stabilization, fluid therapy, and care along with regular exercise opportunities both inside and outside. Rehab M1 was held in a bioactive enclosure, and live-prey tested prior to his release after 54 weeks of inpatient rehabilitation. Mass at soft release = 361 g, average carapace size over the course of study involvement was 9.6 cm × 12.4 cm, annuli = 16.
Rehab M2 was an adult male struck by a lawnmower and first admitted to a separate wildlife rehabilitation facility on 31 August 2018 from a location approximately 68 km northwest of the study area. Rehab M2 received standard wound stabilization and care at this separate facility and was held primarily indoors in a dry aquarium habitat. At the time of transfer to NWCC in late spring of 2020, the EBT was not using its posterior legs for locomotion. Rehab M2 was moved to a bioactive enclosure and regained limb function following a regimen of customized physical therapy sessions begun in fall 2020 and conducted three times per week until his release after 148 weeks in captivity. Mass at soft release = 340 g, average carapace size over the course of study involvement was 9.9 cm × 13.5 cm, annuli = 10.
Rehab F was a juvenile female admitted to NWCC on 15 May 2020 from a location approximately 22 km south of the study area. Rehab F presented with injury of unknown etiology to the anterior right carapace, an inflamed and immobilized right anterior leg, and an older injury to the anterior left carapace. Rehab F was severely emaciated on arrival and received standard wound stabilization, fluid therapy, and care. She also received regular exercise opportunities both inside and outside, as well as a customized physical therapy routine (started fall 2020), plus laser therapy treatments and stimulation of injured limbs with vibrations (started February 2021 in consultation with veterinarians). Rehab F was never kept in a bioactive enclosure due to limited facility capacity and was released after 159 weeks in captivity. Mass at soft release = 313 g, average carapace size over the course of study involvement was 8.8 cm × 11.1 cm, annuli = 11.
All three rehabilitated EBTs were deemed fit for release by qualified veterinarians and certified wildlife rehabilitators prior to participation in the study. Each EBT spent approximately three months in quarantine, were prophylactically dewormed, and tested negative on a standard respiratory panel (Mycoplasma spp., ranavirus, and chelonid herpes) prior to the soft release period to mitigate the potential for disease spread to wild EBT populations.
Wild turtles
Two sympatric wild EBTs, Wild F and Wild M, were selected from the study area for concurrent monitoring. Turtles were found opportunistically on foot and selected for proximity to the soft release pen locations: Wild F (initial mass = 472 g, average carapace size 9.8 cm × 12.6 cm, annuli = 10) was captured approximately 20 meters from Site A, and Wild M (initial mass = 492 g, average carapace size 10.8 cm × 14.1 cm, annuli = 11) was captured approximately 230 meters from Site B. Each wild turtle was selected to match the sex of their paired rehabilitated turtle. Wild EBTs were brought to NWCC for a complete physical exam where they were radio tagged (see Radio Telemetry) before being returned to the study area and released.
Soft release pens
Soft release pen designs were modified from pens used to successfully protect EBTs from predators under similar environmental conditions (Tetzlaff et al. 2020). Two 3 ft × 3 ft × 6 ft soft release pens were constructed from 1.5 inch PVC pipe and enclosed with plastic poultry netting on the top and sides using zip ties. The bottoms were left open to facilitate access to natural habitat. Pen sides were buried to a depth of 6” in order to firmly secure the pens and to deter potential predators (Fig. 1). Pens were monitored for predator and human activity using motion-sensing game cameras for approximately one week prior to and throughout the soft release period.

Fig. 1 Site A soft release pen installation.
Rehabilitated EBTs were released into pens asynchronously. For each approximately 21-day soft release period, a rehabilitated turtle was provided with NWCC turtle diet (~50% produce/leafy greens, ~50% varied protein), mealworms, water, and shelter to supplement the natural mesocosm contained within the enclosure. Food and water were refreshed as necessary (≤ every three days). At the conclusion of the soft release period, each pen was opened by removing the lower poultry netting from one side, at which point the turtles were able to come and go at their leisure.
Radio telemetry
To facilitate post-release monitoring in the field, reptile glue-on radio transmitters (Advanced Telemetry Solutions, model R1860) were attached using waterproof epoxy to the anterior-left quadrant of the carapace of each study turtle with the antennae oriented distally. This attachment location was selected to help minimize interference with mating. Transmitters were attached at NWCC and epoxy allowed to cure for 30 min immediately before rehabilitated or wild EBTs were soft released or returned to the study area, respectively. Wild and rehabilitated turtles were located using standard radio telemetry equipment (folding 3-element Yagi antenna model 13860 and 4 MHz receiver model R410, both from Advanced Telemetry Solutions). Rehabilitated EBTs were located daily during the week following release from the pens, biweekly through the summer, and weekly through the fall and spring. Wild turtles were always located immediately after their rehabilitated counterparts. Turtles were located every other week during winter when travel was less likely due to brumation.
Health monitoring
Once weekly, except during brumation, physical exams were conducted by trained NWCC interns to evaluate EBT health. Field exam results were evaluated by a state-certified wildlife rehabilitator at NWCC. Photos were taken of the turtle in situ, weights were measured to the nearest gram using a calibrated balance scale, and carapace measurements were measured with manual calipers to the nearest 10th of a centimeter. Body condition index (BCI) scores were calculated using weight (g) and carapace width (mm) to estimate fat volume (dePersio et al. 2019), which was used as an indicator of health when compared to an individual turtle’s baseline BCI. In male turtles, eye color was evaluated for saturation using a scale of 1–5 developed by Cerreta et al. (2018). A score of one is indicative of pale pink eye color and a score of five indicates deep red coloration. Eye color in male box turtles is directly correlated with packed cell volume and a low score is a potential indicator of anemia (Cerreta et al. 2018).
Study turtles were also monitored qualitatively in the field for any obvious symptoms of disease, dehydration, or injury as described by Somers et al. (2017). Dehydration was evaluated through visual assessment of skin condition and elasticity. Additional physical exams were conducted as necessary to evaluate any observed declines in EBT health, but handling was kept to a minimum. Gloves and hand sanitizer were used to mitigate potential for disease spread between study turtles. If multiple turtles were released at a site (A or B), the soft release pen was repositioned within that site to further minimize disease transmission. In accordance with permit requirements, both wild and rehabilitated EBTs were temporarily removed from the field if consulting, state-certified wildlife rehabilitators deemed medical intervention necessary.
Brumation periods were determined retroactively for each turtle by evaluating movements. Consistent with other studies, a turtle was considered ‘in brumation’ if it was found in the same location (<1 meter of observed movement) for a period of four consecutive weeks (DeGregorio et al. 2017). The initial brumation date was determined as the first time the turtle was observed in that location; the last date of brumation was determined as the first time a turtle was observed in a new location. Weekly tracking of EBTs continued for at least two months post-brumation to ensure that any symptoms of disease had fully abated and body condition was stable prior to cessation of monitoring.
Data analysis
Each time a turtle was located, Global Positioning System (GPS) coordinates were collected to an accuracy of at least <30 meters using a Garmin eTrex Personal Navigator 12 channel GPS. To quantify the minimum distance that a turtle travelled between observations, the distance in meters from the soft release pen to each GPS coordinate was calculated using the Haversine formula via the geosphere package in RStudio Version 2022.07.0+548. Once all distances were calculated (Supplemental Fig. S1), the median of these distances was determined for each turtle as a measure of dispersal from the soft release pen.
GPS coordinates were then plotted in QGIS Version 3.26, and home range sizes were calculated using 100% minimum convex polygons. This relatively simple method of home range size estimation was chosen to facilitate comparison of results across studies led by other wildlife rehabilitators with potentially limited resources. To account for initial period of dispersal away from the soft release pen, home range sizes were first calculated with all coordinates and then calculated again with only coordinates from the dates after a turtle achieved its median minimum distance from the pen. Both home range size estimates are reported for rehabilitated turtles.
Results
The study period ran from June 2021 through August 2022. Key dates and associated health data are provided (Table 1). All male turtles involved in the study required health interventions of varying severity for symptoms likely related to respiratory infection. All turtles who completed the initial soft release period entered brumation and emerged the following spring.
Rehab M1
Rehab M1 suffered a severe decline in health during the initial soft release period. Symptoms were indicative of respiratory infection and included lethargy, decreased BCI, decreased eye color saturation score (Table 1), mild dehydration, closed pustulant eyes, and discharge from the nose. After 13 days, Rehab M1 was withdrawn from the study never having left the confines of the soft release pen.
Rehab M2
Rehab M2 was selected as a replacement for Rehab M1 and soft released at Site B. Rehab M2 was temporarily removed from the study for a health intervention 53 days after initial release. Symptoms observed over the 14-day period prior to removal were consistent with respiratory infection and included increasingly pustulant eyes, dehydration, lethargy, and later, a sudden and sustained decrease in body condition (Table 1). After 14 days of rehydration and standard care at NWCC, considering improved eye color, symptom abatement, and increasingly stable BCI, Rehab M2 was deemed healthy by certified wildlife rehabilitators and returned to the removal location. Rehab M2 achieved a total brumation time between 130 and 151 days, and subsequent observation revealed no further signs of illness. Rehab M2 traveled a median distance of 825.77 m from the soft release pen location over the course of the study period. Total home range size was 3.41 ha (n = 57), and home range size after accounting for the initial dispersal period was 2.33 ha (n = 39) (Fig. 2).
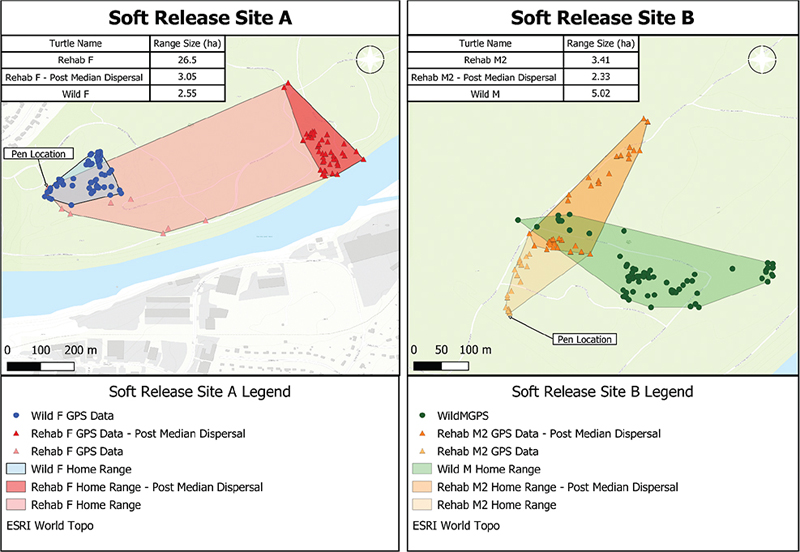
Fig. 2 Maps of Site A and Site B that depict estimated range sizes for study turtles as calculated using minimum convex polygons. Home ranges were calculated both before and after dispersal away from the pen location.
Rehab F
Rehab F was soft released at Site A. Rehab F exhibited no symptoms of illness or injury during the study period, and BCI increased after release (Table 1). Rehab F traveled a median distance of 825.77 m from the soft release pen location and achieved a total brumation time between 137 and 154 days. Total home range size was 26.5 ha (n = 56), and home range size after accounting for the initial dispersal period was 3.05 ha (n = 46) (Fig. 2).
Wild M
After 148 days of observation, Wild M was temporarily removed from the field for a health intervention. In the weeks prior to the intervention, Wild M developed pustulant eyes and lethargy, a sudden and sustained decrease in body condition, and reduced eye color saturation (Table 1). Wild M was deemed healthy after 15 days of rehydration and standard care at NWCC, no longer displayed symptoms and was returned to the removal location. Wild M achieved a total brumation time between 137 and 151 days, and subsequent observation revealed no further signs of illness. Total home range size for Wild M was 5.02 ha (n = 76) (Fig. 2).
Wild F
Wild F exhibited no symptoms of illness during the data collection period and achieved a total brumation time between 151 and 168 days. Total home range size was 2.55 ha (n = 65) (Fig. 2).
Discussion
Although this study involved an extremely limited sample size, we can conclude that soft release of EBTs via penning is a practical strategy that might be leveraged by wildlife rehabilitators. The pens were low-cost, simple to construct, and can be easily relocated to appropriate habitats within and between sites. EBTs who completed the soft release penning period appeared to establish and maintain ranges within the intended release area (Fig. 2) and survived through brumation (Table 1).
Benefits of pens
Results from this limited EBT study align with other reports that soft release penning can support the health of translocated chelonians (Resende et al. 2021). Soft release pens provide a stopgap to identify EBTs unfit for translocation despite inpatient rehabilitation success (as was our experience with Rehab M1). Soft release pens also offer a window to monitor EBTs selected for translocation for as long as necessary. Rehabilitators can adjust the penning period as indicated for an individual EBT based on clinical judgment, weather, turtle behavior, or other variables they believe might affect an individual’s survival. Rehabilitators can also adjust a release location if hazards or predators are regularly observed via game cameras around the pen. Although a 21-day soft release period was sufficient for our study, to our knowledge, there is no standard soft release penning duration for rehabilitated EBTs and it is possible that longer-term penning (Frederick 2009) or repositioning EBTs within the relocation habitat (Poor et al. 2020) may further support successful translocations.
Importance of health monitoring
In this study, we used several objective and subjective metrics to monitor the long-term health of translocated and wild turtles. Eye color scores and BCI were both easily determined in the field with simple measurements, and both proved useful as metrics of turtle health during and after soft release periods. In our study, BCI fluctuated, though steady declines often preceded health interventions and coincided with symptoms of respiratory disease. Rehab F showed the strongest evidence of improved BCI over the course of this study (Supplemental Fig. S2). It is important to note that BCI scores may be inflated in female chelonians due to egg or clutch size (Wallis et al. 1999), and some BCI fluctuations may indicate reproductive activity. This is one limitation of BCI measurements and highlights the importance of contextualizing BCI with other assessments, such as eye color assessments and physical exams.
Range establishment
The establishment of new home ranges among rehabilitated EBTs in this study (Fig. 2) followed a long, linear dispersal pattern from the release site comparable to other studies (Rittenhouse et al. 2007; Attum & Cutshall 2015). Reported home ranges for EBTs vary widely (Cook 2004; Attum & Cutshall 2015) and even within individuals over time (Henriquez et al. 2017). Even after an initial dispersal period was accounted for, the female rehabilitated EBT in this study established a home range comparable, albeit slightly larger, than that of the wild female (3.05 ha vs. 2.55 ha), akin to results from three-toed box turtles (Rittenhouse et al. 2007). Unexpectedly, Rehab M2 established a smaller home range than Wild M (3.41 ha post-dispersal vs. 5.02 ha). Several factors may have contributed to this smaller home range size including effects of the soft release pen, this turtle’s locomotive difficulties or external environmental factors such as the presence of an existing EBT population. Wild EBT population density and sex ratio at the study site is unknown, but habitats may support a greater number of male than female EBTs (Whitehead 2017). The long-term durability of these new home ranges is unknown. It may take several years for a translocated EBT to establish a new home range (Cook 2004), and studies show chelonians demonstrate variable fidelity to release location (Sosa & Perry 2013; McKee et al. 2021).
Post-release considerations
Long-term monitoring is a consideration for any translocation (Poor et al. 2020). Wildlife rehabilitation teams can and should monitor released species with particular focus on individual risk factors and periods of stress within the translocation period. While it has an upfront cost, radio telemetry equipment can be reused (with the exception of the transmitters) to support further monitoring. Based on our experience with this study, translocation via soft release penning is advised for rehabilitated EBTs when coupled with adequate long-term health and location monitoring capabilities including radio telemetry equipment and a capable staff or volunteer team.
Conclusion
Soft release penning provides a unique opportunity to observe health and behavior in a controlled environment prior to release. This early intervention period may aid rehabilitators in choosing viable box turtle candidates for translocation. We advise long-term monitoring post-release to facilitate health interventions if turtles are unable to establish home ranges nearby to the soft release location. Future studies could census existing populations prior to soft release of rehabilitated turtles to determine overall population health, composition, and potential carrying capacity. Further, we recommend future studies that incorporate larger sample sizes to examine the benefits of soft release penning on the long-term health and home range size of translocated, rehabilitated box turtles.
Acknowledgments
National Wildlife Rehabilitators Association
Tennessee Wildlife Resource Agency
Metropolitan Nashville Parks and Recreation
Shelby Bottoms Nature Center
Ontario Turtle Conservation Center (physical therapy guidance)
Veterinary Rehabilitation and Sports Medicine of Tennessee (physical therapy guidance)
Nashville Zoo (for assistance developing telemetry protocols)
NWCC volunteers, interns and staff
Dr. Mike Corwin DVM, Airport Animal Clinic
Melissa Davis, Airport Animal Clinic
Dr. James Talbot DVM, Belle Forest Animal Hospital
References
| Attum O. & Cutshall C.D. 2015. Movement of translocated turtles according to translocation method and habitat structure. Restoration Ecology 23, 588–594, doi: 10.1111/rec.12233. |
| Brown J.D. & Sleeman J.M. 2002. Morbidity and mortality of reptiles admitted to the wildlife center of Virginia, 1991 to 2000. Journal of Wildlife Diseases 38, 699–705, doi: 10.7589/0090-3558-38.4.699. |
| Cerreta A.J., Mehalick M.L., Stoskopf M.K., Dombrowski D.S. & Lewbart G.A. 2018. Assessment of a visual scoring system for identifying and quantifying anemia in male eastern box turtles (Terrapene carolina carolina). Journal of Zoo and Wildlife Medicine 49, 977–982, doi: 10.1638/2018-0045.1. |
| Cook R. 2004. Dispersal, home range establishment, survival, and reproduction of translocated eastern box turtles, Terrapene c. carolina. Applied Herpetology 1, 197–228, doi: 10.1163/157075403323012197. |
| dePersio S., Allender M.C., Dreslik M.J., Adamovicz L., Phillips C.A., Willeford B., Kane L., Joslyn S. & O’Brien R.T. 2019. Body condition of eastern box turtles (Terrapene carolina carolina) Evaluated by Computed Tomography. Journal of Zoo and Wildlife Medicine 50, 295–302, doi: 10.1638/2018-0201. |
| DeGregorio, B.A., Tuberville T.D., Kennamer R.A., Harris B.B. & Brisbin I.L. 2017. Spring emergence of eastern box turtles (Terrapene carolina): Influences of individual variation and scale of temperature correlates. Canadian Journal of Zoology 95, 23–30. |
| Dodd C.K. & Seigel R.A. 1991. Relocation, repatriation, and translocation of amphibians and reptiles: are they conservation strategies that work? Herpetologica 47, 336–350. |
| Frederick N. 2009. Examining the effects of penning on juvenile eastern box turtles (Terrapene carolina carolina). VCU Scholars Compass. |
| Gibbons J.W., Scott D.E., Ryan T.J., Buhlmann K.A., Tuberville T.D., Metts B.S., Greene J.L., Mills T., Leiden Y., Poppy S. & Winnie C.T. 2000. The global decline of reptiles, deja vu amphibians. BioScience 50, 653, doi: 10.1641/0006-3568(2000)050[0653:TGDORD]2.0.CO;2. |
| Henriquez M.C., Macey S.K., Baker E.E., Kelly L.B., Betts R.L., Rubbo M.J. & Clark J.A. 2017. Translocated and resident eastern box turtles (Terrapene c. carolina) in New York: movement patterns and habitat use. Northeastern Naturalist 24, 249–266, doi: 10.1656/045.024.0303. |
| Hester J.M., Price S.J. & Dorcas M.E. 2008. Effects of relocation on movements and home ranges of eastern box turtles. The Journal of Wildlife Management 72, 772–777, doi: 10.2193/2007-049. |
| McKee R.K., Buhlmann K.A., Moore C.T., Hepinstall-Cymerman J. & Tuberville T. 2021. Waif Gopher Tortoise survival and site fidelity following translocation. The Journal of Wildlife Management 85(4), 640–653, doi: 10.1002/jwmg.21998. |
| McKee R.K., Buhlmann K.A., Moore C.T., Allender M.C., Stacy N.I. & Tuberville T.D. 2022. Island of misfit tortoises: waif gopher tortoise health assessment following translocation. Conservation Physiology 10, 1–18. |
| Poor E.E., Spivy A., Rohrbaugh L. & Mullinax J.M. 2020. An ad hoc translocation of urban eastern box turtles (Terrapene carolina carolina). Northeastern Naturalist 27, 631–640, doi: 10.1656/045.027.0403. |
| Resende P.S., Viana-Junior A.B., Young R.J. & Azevedo C.S. 2021. What is better for animal conservation translocation programmes: soft- or hard-release? A phylogenetic meta-analytical approach. Journal of Applied Ecology 58, 1122–1132, doi: 10.1111/1365-2664.13873. |
| Rittenhouse C.D., Millspaugh J.J., Hubbard M.W. & Sheriff S.L. 2007. Movements of translocated and resident three-toed box turtles. Journal of Herpetology 41(1), 115–121, doi: 10.1670/0022-1511(2007)41[115:MOTART]2.0.CO;2. |
| Somers A.B., Matthews C.E. & LaVere A.A. 2017. The box turtle connection: building a legacy. Accessed on the internet at https://boxturtle.uncg.edu/wp-content/uploads/2017/12/BTC-Legacy-2017.pdf on 30 December 2022. |
| Sosa J.A. & Perry G. 2013. Site fidelity, movement, and visibility following translocation of ornate box turtles (Terrapene Ornata ornata) from a wildlife rehabilitation center in the high plains of Texas. Herpetological Conservation and Biology 10(1), 255–262. |
| Tetzlaff S.J., Robinson C.J., Kingsbury B.A., Sperry J.H. & Degregorio B.A. 2020. Predators may lose interest in turtle acclimation pens: implications for translocations using soft release. Chelonian Conservation and Biology 19, 141–144, doi: 10.2744/CCB-1405.1. |
| Tetzlaff S.J., Sperry J.H. & DeGregorio B.A. 2019. Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: a review and meta-analysis. Biological Conservation 236, 324–331, doi: 10.1016/j.biocon.2019.05.054. |
| Tuberville T.D., Clark E.E., Buhlmann K.A. & Gibbons J.W. 2005. Translocation as a conservation tool: site fidelity and movement of repatriated gopher tortoises (Gopherus polyphemus). Animal Conservation 8, 349–358, doi: 10.1017/S1367943005002398. |
| van Dijk P.P. 2011. IUCN red list of threatened species: Terrapene carolina. IUCN Red List of Threatened Species. |
| Wallis I.R., Henen B.T. & Nagy K.A. 1999. Egg size and annual egg production by female desert tortoises (Gopherus agassizii): the importance of food abundance, body size, and date of egg shelling. Journal of Herpetology 33(3), 394–408, doi: 10.2307/1565636. |
| Whitehead V. 2017. Population dynamics of the eastern box turtle (Terrapene Carolina carolina) in the Maryville college woods, Maryville, Tennessee. Senior study. Maryville, TN: Maryville College. |