CASE REPORT
Papillomatosis in a raccoon (Procyon lotor)
Alexandra Sanz, VMD,1 Erica Miller, DVM,2 Nikki Harley3 & Laura Coffee, MPH, DVM, DACVP4
1Atlasvet, Washington, DC, USA;
2Wildlife Futures Program, University of Pennsylvania, Kennett Square, PA, USA;
3Royal Veterinary College, London, UK;
4Zoetis, Inc., Annapolis, MD, USA
Abstract
Papillomaviruses are species-specific double-stranded DNA viruses with at least 50 species of mammals. Although well-studied in humans, papillomaviruses in the raccoon (Procyon lotor) are not well characterized, and few cases have been published. Therefore, when the disease is encountered in a rehabilitation center, definitive diagnosis and treatment in the raccoon can be unclear. This case study outlines the characteristic gross and histologic lesions of an affected individual to facilitate identification and treatment of this disease in the future. In the case of this juvenile raccoon, ulcerated exophytic lesions on the bridge of its nose and right forelimb digit were seen grossly, with the presence of koilocytes and hyperkeratosis observed histologically. These findings are consistent with other documented cases of papillomavirus in raccoons and canines. Affected raccoons do not require extensive treatment, as raccoon papillomatosis is considered self-limiting.
BIO
Alexandra Sanz is a veterinarian with Atlasvet in Washington, DC. She handled this case while a veterinary student at the University of Pennsylvania School of Veterinary Medicine.
Nikki Harley is a second-year veterinary student at the Royal Veterinary College and has been a summer intern and seasonal employee at Mercer County Wildlife Center for six years.
Erica Miller is the Field Operations Manager with the Wildlife Futures Program and an Adjunct Associate Professor of Wildlife Medicine at the University of Pennsylvania School of Veterinary Medicine. She volunteers at Mercer County Wildlife Center.
Laura Coffee is a Surgical Pathologist with Zoetis, Inc., Annapolis, MD. She read the histopathology on this case when she was with Antech Diagnostics.
Keywords
Procyon lotor; raccoon; papilloma virus; papillomatosis
Abbreviation
COPV: canine oral papillomavirus
FdPV: feline papillomavirus
HPV: human papilloma virus
PV: papillomavirus
PCR: polymerase chain reaction
Citation: Wildlife Rehabilitation Bulletin 2022, 40(1), 22–26, http://dx.doi.org/10.53607/wrb.v40.255
Copyright: Wildlife Rehabilitation Bulletin 2022. © 2022 A. Sanz et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Correspondence to: Erica A. Miller, DVM, 1250 Corner Ketch Road, Newark, DE 19711 USA. E-mail: drsanz@atlasvetdc.com
Accepted: 7 September 2022; Published: 7 October 2022
Disclosure statement and funding: No potential competing interest were reported by the authors.
This case study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Introduction
PVs are double-stranded DNA viruses that affect many species of animals including humans. They have the potential to cause cancer by integrating into the host genome; however, this is rare in animals and is mainly seen with HPV. Raccoons can acquire a species-specific papillomavirus, but that viral species is not well-documented, and little is known about its pathogenesis.
Only two cases of raccoon PV are documented in the scientific literature. One involves a field trial for the V-RG® rabies vaccine in Virginia in 1995, in which two out of 53 raccoons were positive for papillomavirus (Hamir et al. 1995). In another study, an adult raccoon was captured in Toronto, Canada, and tested positive for papillomavirus (Rector et al. 2005). In both cases, the lesions existed on the front feet of the raccoons.
Raccoon PV belongs to the Lambda-papillomavirus genus, making it related to both COPV and FdPV (Rector et al. 2005). Using information from one of the more well-studied Lambda-PVs, COPV, we can better understand raccoon papillomatosis. COPV lesions typically are exophytic verrucous warts in the oropharynx, meaning that they protrude out of the mucous membrane and have a cauliflower-like appearance. Lesions start to develop at a young age, and typically 75–80% regress spontaneously within weeks to months. COPV is transmissible by direct or indirect contact, and once a dog acquires the papillomavirus, it is typically protected against reinfection (Ghim et al. 2000). Lesions in dogs can linger and potentially become carcinogenic; however, this has not been noted in raccoons (Sundberg et al. 2004).
The pathogenesis of raccoon papillomavirus is unknown, but because of its similarity to COPV in both genetics and clinical signs, the general features of COPV can be used as a template for treatment, release, and prevention in raccoons. Clinical similarities include benign exophytic areas in the skin, typically found on the feet in raccoons as opposed to the oral mucosa in canines. Just like COPV, raccoon PV lesions have been noted in juvenile animals and are believed to regress in a couple of weeks (Sundberg et al. 2004).
Diagnosis of papillomavirus includes recognizing gross lesions based on their anatomical location and appearance. A simple excisional biopsy of the lesion can be obtained to give a presumptive diagnosis on histopathology. Immunohistochemistry can be used to give a definitive diagnosis. While not utilized typically outside a research laboratory setting, monoclonal and polyclonal antibodies can be used to establish if the virus present in the lesion is responsible for a productive infection, and thus the true etiological source of the lesion. Differential diagnoses for this lesion include poxvirus, herpesvirus, adenovirus, and retrovirus (Sundberg et al. 2004); therefore, it is important to identify PVs correctly for contagious or zoonotic reasons. In the case of COPV, the virus is transmissible only to other canines and does not pose a threat to humans. COPV and raccoon PV are considered self-limiting, so treatment is not necessary in most cases (Jun et al. 2010).
History and signs
A juvenile raccoon recovered from a citizen’s backyard in New Jersey presented with exophytic firm erosive lesions on the bridge of the muzzle and dorsal right front digit at Mercer County Wildlife Center in New Jersey (Figs. 1 and 2). The raccoon was anesthetized, and an excisional biopsy of the right second digit was taken and sent to Antech Diagnostics (Levittown, PA) for histopathology analysis.

Fig. 1 Ulcerated firm skin lesion on the bridge of muzzle.

Fig. 2 Ulcerated exophytic lesion on the bridge of muzzle and dorsal aspect of the right second digit, where biopsy was taken.
Diagnosis
A key feature of papillomavirus is the presence of koilocytes in the stratum spinosum and stratum granulosum, the second and third layers of the epidermis. Koilocytes are cells with a small, dark basophilic nucleus and cytoplasmic clearing that do not take up a stain. Other characteristic microscopic lesions of papillomatosis include hyperkeratosis, intranuclear inclusion bodies, epithelial dysplasia, and a hyperplastic stratum granulosum (Hamir et al. 1995; Rector et al. 2005).
Histopathological findings from the toe biopsy were consistent with raccoon papillomatosis. In Fig. 3, the biopsy contains koilocytes (indicated by the green arrows). These cells are mainly present in the stratum spinosum. No inclusion bodies are noted, but they can regress in later stages of infection. In Fig. 4, superficial epidermal proliferation supported by fibrocollagenous cores and parakeratotic hyperkeratosis is noted, indicative of a viral papilloma infection. In addition, there are multifocal erosions with neutrophils, indicating a superficial bacterial pyoderma, typically seen in PVs in wild animals. Further analysis to rule out dermatophytes was performed and tested negative.
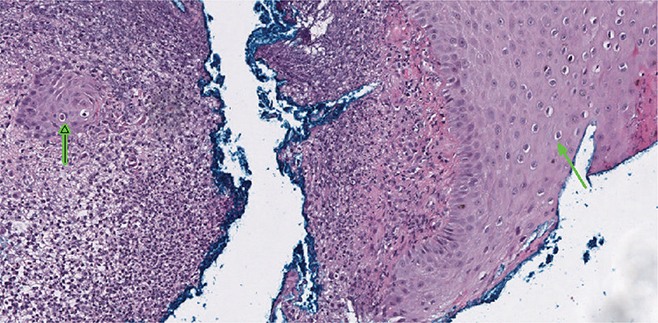
Fig. 3 Microscopic lesion of the biopsy of the right dorsal second digit. Koilocytes, a hallmark feature of papillomavirus, are indicated by the green arrows. They are characterized by cytoplasmic clearing and a small dark nucleus.
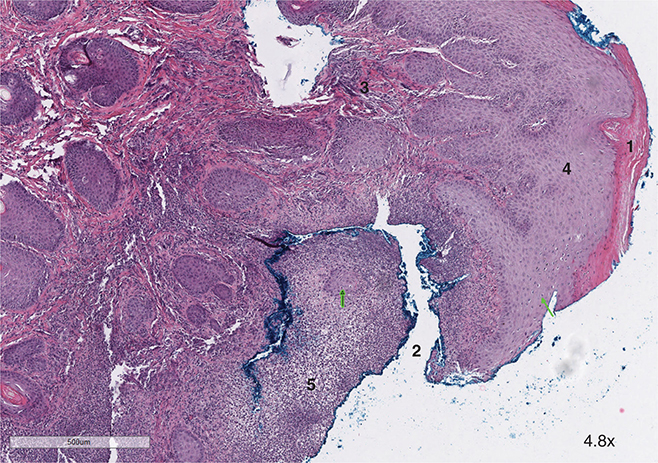
Fig. 4 Characteristic features of papillomavirus include (1) parakeratosis, (2) erosions, (3) fibrocollagenous cores, and (4) papillary-type hyperplasia. Features indicative of a secondary superficial pyoderma due to the viral infection include neutrophilic crust (5).
Confirmation of raccoon papillomavirus can also be performed via immune-histochemical tests to look for the presence of papilloma antigens in koilocytes (Rector et al. 2005) or a PCR to determine the presence of the virus. These tests were not performed in this case.
Treatment and prevention
COPV lesions (related to raccoon papilloma lesions) are commonly self-limiting and most will spontaneously regress. Although there have been reports that the canine virus can become recurrent, it is rare and usually seen in immunocompromised patients (Sundberg et al. 2004). There have been no reports of raccoons developing neoplasms or recurrent infections. Transmission is by direct contact, so it is important to keep affected animals separated until release to prevent spread to other raccoons in the facility. The virus can remain on the premises for years. The efficacy of different disinfectants has not yet been evaluated in non-human PVs, but research on HPV suggests aldehyde-based disinfectants are most effective (Egawa et al. 2021). A quaternary ammonium disinfectant with viricidal properties was used daily to clean both indoor and outdoor enclosures where the raccoon was held. Once the raccoon was released, the outdoor enclosure’s substrate was removed, and the entire enclosure was cleaned with a 1:30 dilute bleach solution. The cage was not used for the rest of the rehabilitation season to prevent possible exposure of other raccoons to the papillomavirus.
Treatment and control of PVs are not needed in free ranging wildlife; however, the presented raccoon was held for treatment with Collasate® silver (PRN Pharmacal Pensacola, FL) for the secondary pyoderma and released once the ulcer was healed (Fig. 5). Collasate® silver is a topical gel including a Type I collagen hydrolysate and silver oxide. The gel was applied to the nose lesion and biopsy site on the digit only one time to manage the secondary pyoderma and promote wound healing. If a more extensive secondary pyoderma was evident in the presenting raccoon, a systemic antibiotic with no known species contraindications would be appropriate (e.g., Ceftiofur). The papilloma lesion was not fully regressed at the time of release, but indications of regression include infiltration of lymphocytes, hyalinization of the connective tissue, and eventual sloughing of the tumor (Sundberg et al. 2004). Therefore, some scarring may be present once regressed.

Fig. 5 Juvenile raccoon just prior to release. Ulcerations have healed, and papilloma has partially regressed.
Summary
A juvenile raccoon presented with ulcerated exophytic lesions on the bridge of its nose and second digit of the right forelimb. Toe biopsy was taken and sent to histopathology for a definitive diagnosis. Although no inclusion bodies were seen in the tissue, the diagnosis of papillomatosis was made based on the presence of koilocytes, a parakeratotic hyperkeratosis, and a hyperplastic stratum spinosum. Papillomavirus lesions are believed to be self-limiting; therefore, the only treatment necessary was medical management of the ulcerated skin. Once the ulcer was healed, the raccoon was ready for release. Papillomavirus lesions in raccoons are typically found on the digits, are exophytic, and will regress within weeks. Since it is not necessary to hold the raccoon in the facility while the lesion regresses, it is important to diagnose both gross and microscopic characteristics of the lesions to create a better plan for treatment and release.
References
| Egawa N., Shiraz A., Crawford R., Saunders-Wood T., Yarwood J., Rogers M., Sharma A., Eichenbaum G. & Doorbar J. 2021. Dynamics of papillomavirus in vivo disease formation & susceptibility to high-level disinfection-implications for transmission in clinical settings. eBioMedicine 63, 4–11, doi: 10.1016/j.ebiom.2020.103177. |
| Ghim S., Newsome J. & Bell J. 2000. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Experimental and Molecular Pathology 68(3), 147–151, doi: 10.1006/exmp.1999.2298. |
| Hamir A.N., Moser G., Jenson A.B., Sundberg J.P., Hanlon C. & Rupprecht C.E. 1995. Papillomavirus infection in raccoons (Procyon lotor). Journal of Veterinary Diagnostic Investigation 7(4), 549–551, doi: 10.1177/104063879500700424. |
| Jun D., Yi G., Na T., Yipeng J., Rui Z., Degui L. & Guozhong Z. 2010. Canine oral papillomavirus infection: clinical course, pathology, L1 gene and NCR2 gene sequencing. Israel Journal of Veterinary Medicine 65(3), 111–116. Accessed on the internet at http://www.ijvm.org.il/sites/default/files/5_canine_oral_papilloma.pdf on 20 August 2022 |
| Rector A., Van Doorslaer K., Bertelsen M., Barker I.K., Olberg R.A., Lemey P., Sundberg J.P. & Van Ranst M. 2005. Isolation and cloning of the raccoon (Procyon lotor) papillomavirus type 1 by using degenerate papillomavirus-specific primers. Journal of General Virology 86, 2029–2033, doi: 10.1099/vir.0.80874-0. |
| Sundberg J.P., Van Ranst M. & Jenson A.B. 2004. Papillomavirus infections. In E.S. Williams & I.K. Barker (eds.): Infectious diseases of wild mammals. Pp. 223–231. Ames, IA: Iowa State University Press. |