ARTICLE
Physiological impacts of wildfire exposure on four American black bears
Veronica Gordon, BS, CWR, LVT
Abstract
A substantive wildfire season in Eastern Washington in July and August 2021 led to the admittance of four American black bears (Ursus americanus) to PAWS Wildlife Center for wildfire-related injuries. The animals each suffered burns of first to fourth degree classifications in addition to trauma related to smoke inhalation. In one case, the patient experienced either permanent or transient physiologic changes to the respiratory, cardiovascular, and nervous systems. The basis for anticipating and managing trauma in wildfire-affected wildlife in these cases was extrapolated from published literature in small animal veterinary medicine. The purpose of this article is to help correct the absence of formal literature regarding the physiological impact of wildfires on wildlife in a rehabilitative setting.
BIO
Veronica is a licensed veterinary technician who has been working with wildlife, exotic, and domestic animals in varying capacities since 2012. Veronica joined the staff at PAWS Wildlife Center as a Licensed Veterinary Technician in early 2021 and was previously employed by Cape Wildlife Center and MSPCA Angell for several years in her home state of Massachusetts. Veronica was involved in the direct management of the animals referenced in this paper and presented on this topic at both the Washington Wildlife Rehabilitators Association and Wildlife Rehabilitators Association of Massachusetts conferences in 2021 and 2022, respectively.
Keywords
Wildfire; black bear; Ursus americanus; burn; wound management
Abbreviation
WDFW: Washington Department of Fish & Wildlife
CRI: continuous rate of infusion
IV: intravenous
CBC: complete blood count
SSD: silver sulfadiazine
NSAID: non-steroidal anti-inflammatory drug
Citation: Wildlife Rehabilitation Bulletin 2022, 40(1), 8–16, http://dx.doi.org/10.53607/wrb.v40.249
Copyright: Wildlife Rehabilitation Bulletin 2022. © 2022 V. Gordon. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Correspondence to: Veronica Gordon, PAWS Wildlife Center, Lynnwood, WA, USA. E-mail: vgordon@paws.org
Accepted: 8 September 2022; Published: 7 October 2022
Disclosure statement and funding: The authors report no conflict of interest.
Introduction
With an increasing occurrence of wildfires in Washington state between 2010 and 2020 (WA DNR 2020), there is a direct increase in the potential for wildlife rehabilitators to admit wildlife suffering from trauma associated with wildfires. In recent literature, it has been established that 80% (49/61) of surveyed wildlife rehabilitation centers internationally between 2015 and 2018 have admitted burned wildlife (Butkus et al. 2021). The management of emerging concerns in wildlife rehabilitation often suffers from a lack of published literature to draw from; this was the case when addressing physiological impacts for wildlife being admitted to rehabilitative care post-wildfire exposure. This deficit can be addressed through extrapolating information from adjacent facets of veterinary medicine as well as from direct experience in the field.
Between 27 July 2021 and 27 August 2021, four American black bears (Ursus americanus) were admitted to PAWS Wildlife Center through the WDFW. All four patients were determined to require medical intervention by WDFW officers through observed or reported injury in active wildfire regions. Of the four bears, three were cubs estimated to be around six months of age, and one was a yearling. After an initial evaluation and post-exam discussion with WDFW, the yearling bear (bear “D”) was euthanized due to the severity of the trauma it had sustained.
Background and case reports
Sedation for capture in the field was managed and administered by WDFW officers, typically utilizing BAM™ (butorphanol, azaperone, and medetomidine; ZooPharm Inc., Laramie, WY). The animals were then admitted to PAWS within 24 hr of capture. A 24-hr grace period post-admit was given, as per the direction of PAWS veterinarians, before chemical sedation was attempted again. These animals were sedated most frequently through the use of a pneumatic dart gun with either Telazol™ (tiletamine, zolazepam; Zoetis Inc, Parsippany, NJ) used on its own or a multimodal combination of butorphanol, midazolam, and ketamine. After chemical sedation, gas anesthesia (isoflurane; Covetrus, Portland, OR) was needed at variable levels for each patient in the initial stages of burn management. One patient required a ketamine CRI intraoperatively to appropriately manage pain.
The initial debridement and evaluation procedure for each patient took roughly two hours. An intraoperative temporary IV catheter was placed in two cases to provide supportive fluid therapy as these animals were in more critical condition; otherwise, subcutaneous fluid administration was the method of choice for rehydration in the patients considered to be more stable. Preliminary diagnostics were performed for each patient during the intake exam in the form of a CBC, chemistry, and radiographs. A reference laboratory was used for blood work submission for the three bears whose care extended beyond the initial exam, and all values were considered within normal limits for all patients. Radiographs were submitted for formal interpretation only if overt concerns were observed upon initial review, and both lateral and dorsoventral positioning were used for whole-body radiographs of each patient.
Vital statistics information (ECG, capnography, SpO2, temperature, mucous membrane color, etc.) was collected throughout the procedure for routine monitoring and was considered to be within normal limits. Tachycardia and tachypnea were noted intermittently in conjunction with painful stimuli and mostly corrected in those cases with adjustment of anesthetic depth. During the intake procedures, each patient experienced difficulty thermoregulating while under anesthesia. Initial rectal temperatures dropped steadily in the first 15 min of procedure times, typically within a 2 to 3°F variation. Thermoregulatory deficits often corrected with the addition of supplemental heat, and continuous monitoring of rectal temperature was necessary. Multiple forms of heat supplementation were required in some cases and were provided through a combination of a recirculating hot water blanket, heating pad, warming discs, warm parenteral fluids, and warmed blankets.
When assessing and treating patients who have survived a wildfire, there are many cascading physiologic affects that can impact a patient’s ability to survive in both an acute and chronic sense. An animal has the potential to experience transient or permanent damage to the integumentary, cardiovascular, respiratory, and neurologic systems. The rehabilitative care needs for animals that have survived a wildfire event change most significantly when addressing nutrition “because of their fragile metabolic state, the importance of adequate nutrition cannot be overemphasized in patients with healing burn wounds” (Garzotto 2014). Subsequently, it is recommended that “the diet should be high calorie and high protein” to compensate for the presumed hypermetabolic state an animal is in post-wildfire trauma (Garzotto 2014). The patients in rehabilitative care at PAWS were given such a diet for the first four weeks of rehabilitation as the greatest amount of healing occurred during this period.
Various additional husbandry adjustments were necessary to accommodate the patients’ medical needs while in care. Historically, large livestock tubs would be used as a water source for bears at PAWS. However, due to the concern for maintaining dry bandaging, water sources were reduced to five-gallon buckets which were secured against a door or wall to allow only the patient’s head to contact the water source. The use of fine bedding material, such as wood shavings, was avoided to aid in maintaining good wound hygiene. Other husbandry considerations included activity restriction, motion capture video monitoring, and providing engaging and safe enrichment to enhance compliance with bandages.
When addressing the medical management of a burned patient, antibiotic use is not recommended from the start as burns are considered “sterile or colonized only by superficial bacteria in the first 24 hours” (Hedlund 2007). Superficial bacteria proliferate and invade deeper tissue within four to five days of injury, at which time most organisms are identified as gram-positive cocci, the wound site is later thought to be colonized with gram negative bacteria such as Pseudomonas spp. (Hedlund 2007). Wild animals are unlikely to be admitted within the first 24 hrs of burn injuries, so there is a greater potential for introduction of foreign materials into burn sites. Ideal bandage hygiene may not be able to be maintained in some cases, meaning that antibiotic therapy may be necessary. Poor bandage hygiene may be due to inability to physically handle a large or fractious patient at the frequency recommended for bandage changes, damage caused to a bandage by the patient or rehabilitative environment (water exposure, wound contamination via bedding, licking/chewing by patient, etc.) or highly exudative nature of wounds.
There are three main topical therapies recommended when managing burn injuries: SSD cream 1%, medical grade honey, and aloe vera gel. All three medications have great benefit to thermal burns but have restrictions for use with regard to the stage of healing. The use of tilapia skin on burn sites has also been known to be a successful form of topical burn therapy used in wildlife medicine, “the University of California Davis and the California Department of Fish and Wildlife treated three black bears suffering full thickness paw pad burns with specially prepared tilapia skin bandages” (Butkus et al. 2021). The use of tilapia skin in this instance was extrapolated from human medicine, published information in veterinary medicine is not currently present on this approach (Butkus et al. 2021).
SSD as a topical therapy choice is “effective against most gram-positive and gram-negative bacteria and most fungi. It also serves as an antimicrobial barrier, can penetrate necrotic tissue, and enhances wound epithelialization” (Hedlund 2007). As SSD can penetrate necrotic tissue “it is frequently the dressing of choice for burn wounds, since it can penetrate an eschar” (Maxwell & Bennett 2020). There is opportunity to use SSD in a sustained release form, as silver impregnated foam, which is described later in this section as well as shown in use on Bear “A” at PAWS (Fig. 1). SSD “has been shown to have negative effects on fibroblasts and on wound contractions”, meaning its use should be discontinued when fibroblasts arrive roughly four days into the healing process (Maxwell & Bennett 2020).

Fig. 1 Bear “A” with silver foam sutured to the dorsal muzzle, aural bandaging, and peripheral limb bandaging.
Medical grade honey (e.g., Manuka) can reduce edema and inflammation, has antimicrobial effects, enhances wound debridement, and promotes granulation tissue and epithelialization (Hedlund 2007). Medical grade honey increases collagen content and “contains a wide range of amino acids, vitamins, and trace elements in addition to readily assimilable sugars that stimulate tissue growth” (Hedlund 2007). Honey becomes diluted by exudates, meaning bandage changes are recommended one to three times daily, the exact frequency of which being contingent upon the exudative nature of the wound (Maxwell & Bennett 2020). When there is the presence of healthy granulation tissue and/or epithelialization, it is recommended to discontinue the use of this topical therapy (Hedlund 2007). It should be noted that there is the potential for the presence of “Clostridium botulinum spores that may be present in unsterilized honey” available for purchase in grocery stores, and for this reason, it is not recommended for use (Maxwell & Bennett 2020).
Aloe vera gel is considered to be the topical therapy of choice in early burn injury, “healing of thermal burns treated with aloe vera gel has been proven superior to those dressed with SSD in early wound management” (Maxwell & Bennett 2020). However, the use of aloe vera “on full-thickness wounds is discouraged because of its anti-inflammatory effects” (Hedlund 2007). As discussed earlier in this section, Pseudomonas spp. are some of the bacteria known to colonize burn sites, and aloe vera gel has antimicrobial activity that is specifically listed against Pseudomonas aeruginosa (Hedlund 2007). Aloe vera gel can inhibit fungal growth, helps maintain vascular patency, penetrates and anesthetizes tissue, and promotes wound healing through potentially stimulating fibroblast replication as well as epithelial growth (Maxwell & Bennett 2020).
The initial management and bandaging of the burn sites of the animals admitted to PAWS varied based on severity, location, consideration of patient mentation, and anticipated natural behaviors. Each patient’s paw pads had devitalization of 30–75% of the surface area, which significantly recovered in the first two months of rehabilitation. Each patient also had incidental anatomic change from thermal injury resulting in a melted appearance to the tips of the nails, which did not negatively impair nail function in three of the four cases, the fourth case with the most severe nail trauma being Bear “D.” The burn sites were first coated with sterile lubricating jelly for full thickness burns or aloe vera gel for more superficial sites and allowed to sit for several minutes to help hydrate the affected tissues and to clarify margins of healthy tissue. The physical act of debridement was performed using atraumatic forceps only on areas where affected tissue lifted with ease, were clearly necrosed, or had eschars present. Eschars (pictured in Fig. 8 in the Burn Classification section) are the residue of skin elements that have been coagulated by heat, mainly denatured collagen fibers, that will serve as a source of infection if not removed (Hedlund 2007).
After debridement, the areas were flushed extensively with a warmed isotonic solution (0.9% sterile saline or lactated Ringer’s solution) to clean the areas of further debris presence, help promote wound nutrition through the use of isotonic solutions and further hydrate the burn sites. As each patient’s ability to thermoregulate was compromised, warmed flushes were used to support maintenance of appropriate body temperature. Once the affected tissues were considered cleaned and suitable for bandaging, they were gently dried with surgical towels, which provided an even non-abrasive surface and then coated with a topical therapy of choice. The specific topical therapy choice varied based on burn severity and depth for each patient, and even each individual burn site, but was one of the three aforementioned topical medications.
After application of the applicable topical therapy, the paws were dressed beginning with non-adherent sterile padding between the digits, specifically to encourage increased contact time between the topical therapy and burn sites as well as discourage digit fusion. Following sterile padding placement, two inches of 4 × 4 non-woven gauze pads were then placed on the plantar or palmar surface as padding for pressure and pain relief. Roll gauze was then used to secure the primary layers and extended halfway up the antebrachium or tibia, followed in the same pattern with Vetrap™ (3M, St. Paul, Minnesota). Finally, the bandages were covered with Elastikon™ (Johnson & Johnson, New Brunswick, New Jersey) on the dorsal and ventral plantar or palmar surface and at the proximal part of the bandage to reduce the chance of the bandage slipping or being removed (Fig. 1).
One cub, a 6.99 kg female estimated to be within six months of age (to be referred to as Bear “A”), had sustained first to second degree burns to the dorsal muzzle. The burns to the muzzle were managed with a silver foam pad secured in place with stay sutures (Fig. 1). In later iterations, the rostrum was managed with a modified tie over bandage using 3 × 3 non-woven gauze placed as a cover over the silver foam followed by tie-over roll gauze. The pinnae on this patient had also sustained bilateral second-degree burns, which were managed using Telfa™ (Covidien, Mansfield, MA) pads ventrally and dorsally to sandwich the remaining pinnae post-debridement in SSD. The ears were then manipulated to lie flatly and secured with roll gauze, Vetrap, and finally elastikon.
Burn management was performed every 48 hrs for the first week post-intake. The physical debridement was only performed in the first two to three procedures, contingent upon each patient’s individual needs, as tissues declared and began exhibiting granulation beds within the first week. Burn management was then graduated to every 72 hrs for the second week post-intake in three of the cases. Two of the patients, Bear “B” and Bear “C,” required an additional week of bandaging past the second week due to the severity of the initial trauma and presence of topical infection, which was addressed with systemic antibiotics. Each patient was then observed through video or brief visual monitoring for two more weeks post-bandage removal with no further concern regarding soft tissue trauma.
The second and third American black bear cubs, to be referred to as Bear “B” and Bear “C”, respectively, were 13.9 kg and 16.2 kg males estimated to be six months of age. Bear “C” presented with tremors but had normal mentation and ambulation otherwise, and bear “B” presented with normal neurologic status. On physical exam, no trauma to the head or dorsum beyond singed fur and ash debris was appreciated on either patient. An ophthalmic exam noted mild bilateral conjunctival inflammation suspected to be related to irritation from smoke in both patients. The eyes were subsequently flushed using ophthalmic solution for one to two minutes to ensure that no ash debris was present.
Bear “C” and bear “B” both, respectively, had mainly second or third degree burns to the plantar or palmar surface of each foot, with burns extending to the first cranial quarter of the dorsal surface of each forelimb and the majority of the dorsal surface to each hindlimb. The paw pads of each foot were severely cracked and suspected to be devitalized based on the presence of lifting tissue and burn severity. Aside from the paw pads, the most severe areas of burns were located interdigitally, with mild to moderate anatomic change from heat exposure present on the tips of the nails of each foot. On bear “C,” the right hind foot had focal fourth degree burn sites to D4 and 5.
Regarding the presence of tremors observed from bear “C,” no discernable pattern was noted in relation to their occurrence. The tremors were evaluated via video monitoring by a board-certified neurologist and thought not to be associated with seizure activity. The tremors appeared exacerbated by stress and at times looked more specifically to be intention tremors. By the second week post-admission, the tremors were no longer noted. Bear “C” next began to exhibit respiratory change in week two after resolution of the neurologic abnormalities.
Bear “C” experienced tachypnea and dyspnea for periods ranging from brief episodes of panting for a few minutes to prolonged open mouth breathing within the period of an hour. At its inception, the respiratory episodes presented during the late afternoon or evening but were not preceded by any events or stressors otherwise or noted at any repeatable pattern beyond time of day. Radiographically, the patient had evidence of pneumonitis and pulmonary edema on intake (Fig. 2). Shortly after the onset of respiratory abnormalities in week two, a second set of radiographs (Figs. 3 and 4) showed right-sided cardiomegaly with progressed interstitial pattern as well as a healing or healed rib fracture when reviewed by a board-certified radiologist.
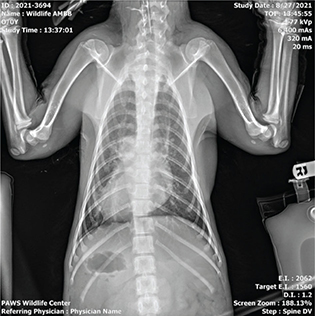
Fig. 2 Dorsoventral radiograph of bear ‘C’ taken on admission showing evidence of pneumonitis and pulmonary edema.
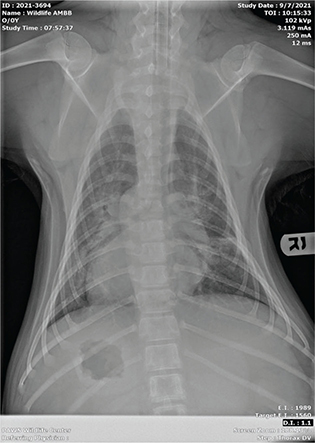
Fig. 3 Dorsoventral view of the second set of radiographs of bear ‘C’, showing right-sided cardiomegaly with progressed interstitial pattern and healing/healed rib fracture.
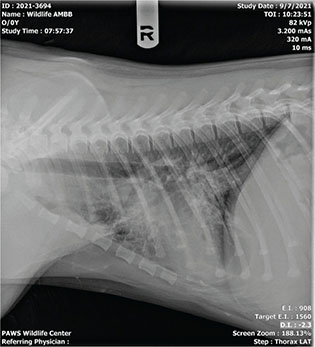
Fig. 4 Lateral view of the second set of radiographs of bear ‘C’ showing right-sided cardiomegaly with progressed interstitial pattern and healing/healed rib fracture.
The cardiac change and worsening condition of the lung field of Bear “C” were suspected to be causative of the respiratory status decline. Empiric treatment with furosemide was initiated at this time. A third set of radiographs (Fig. 5) taken 11 days after the second set showed resolution of cardiac changes and improvement of pneumonitis, interstitial pattern, and pulmonary edema. An echocardiogram performed by a board-certified cardiologist on the same date as the third set of radiographs also corroborated return of normal heart structure and function. The patient experienced respiratory abnormalities from week two to five, after the resolution of the respiratory changes and completion of empiric treatment, the episodes of dyspnea and tachypnea did not return.
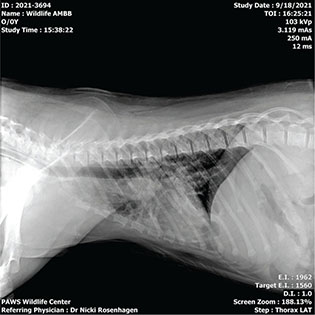
Fig. 5 Lateral view of the third set of radiographs of bear ‘C’ showing resolution of cardiac changes and improvement of pneumonitis/interstitial pattern/pulmonary edema.
Bears “A,” “B,” and “C” were treated prophylactically with enrofloxacin and amoxicillin-clavulanate for antibiotic coverage beginning at intake, as it was known that ideal bandage hygiene would not be able to be maintained. Antibiotic use was continued for a period of three weeks total from intake for bear “B” and bear “C,” as evidence of topical infection was also present. Regarding pain management, each patient was given buprenorphine SR and tramadol for the first two weeks. Gabapentin was prescribed for nerve-associated pain for all three patients as well as additionally for possible seizure activity for bear “C” prior to being ruled out by the neurologist after the first week. Meloxicam was given for the first three weeks in care for all three bears but was replaced by carprofen after for an additional two weeks for bear “C.” The use of a NSAID was only pursued after each patient was rehydrated due to concerns for the presenting hydration status and potential for negative impact on renal function.
Pathophysiology
When an animal is exposed to a fire, the primary emergent concerns are most often associated with smoke inhalation injury (Epstein 2019). Smoke inhalation injury carries two components, thermal injury to soft tissue and inhalation of toxic gases. With wildfires, “the predominant toxic gas is carbon monoxide, which is readily generated from the combustion of wood and other cellulosic materials” (Terrill et al. 1978). When carbon monoxide is inhaled, it binds to hemoglobin, which is the main component of blood that is responsible for oxygen transport from the lungs to the rest of the body.
When carbon monoxide binds to hemoglobin, it is typically by about 50% (Epstein 2019). This translates to oxygen being transported throughout the body at half the normal volume. This reduction in oxygen transport can cause hypoxemia or low concentration of oxygen in the blood, which systemically affects oxygen saturation and can lead to hypoxic shock. The top concerns regarding systemic dysfunction from hypoxemia would lie with the cardiovascular, respiratory, and nervous systems. Reduction in oxygen availability can have short-term and long-term effects as it cascades to disrupt normal bodily functions on many levels, its chronicity being contingent upon the severity of carbon monoxide toxicity.
When discussing the nervous system, manifestations of hypoxemia are typically delayed by days or weeks, and usually present as deficits in gait or ataxia with its permanency declaring over time (Epstein 2019). Regarding respiratory system effects from hypoxemia associated with acute lung injury, “physical examination routinely discloses tachypnea, dyspnea, increased abdominal effort, crackles, and harsh lung sounds” (Carpenter et al. 2001). Respiratory change is often appreciable on intake but can take 24 hrs to present (Jasani 2014). Cardiovascular function compromised by smoke inhalation and external burn injury “tends to normalize quickly in uncomplicated cases, but complicated cases are more likely to show a range of cardiovascular abnormalities that persist for a longer period” (Jasani 2014). Cardiovascular changes clinically present as tachycardia, arrhythmias, hypotension, systolic dysfunction, and possible trauma to the myocardium associated with carbon monoxide toxicity (Jasani 2014). Congestive heart failure has also been observed in cats with severe burn injury in small animal medicine (Sharpe et al. 2020).
If an animal is presenting very soon after fire exposure, administering 100% oxygen is known to reduce carbon monoxide’s half-life to 40–80 min as opposed to four to six hours in room air (Epstein 2019). Unfortunately, carbon monoxide toxicity is not likely to be readily diagnosed in a wildlife rehabilitation facility. Utilization of pulse oximetry and arterial blood gas evaluations are not considered reliable methods of diagnosis, co-oximetry is the recommended diagnostic tool and is currently limited in its availability in small animal medicine (Epstein 2019). Regardless of confirmation of carbon monoxide toxicity, oxygen therapy can still be of great benefit to animals who have survived a wildfire considering the potential for disruption in systemic oxygen availability.
When smoke is inhaled, the heat associated with smoke is most often dissipated in the upper airway, leading to thermal injury (Epstein 2019). In severe cases, thermal necrosis and tracheal stricture can occur from thermal injury to the upper airway (Epstein 2019). Once the soft tissue becomes damaged, factors such as swelling, mucus, and edema can act as a plug that could further obstruct the airway or compromise an animal’s ability to breathe (Pashmakova 2016). Given this information, the severity of lower airway trauma can in part be associated with the severity of the trauma occurring to the upper airway. Lower airway trauma is most often associated with the degree of toxic gases being inhaled, again as the thermal injury component of smoke inhalation has typically dissipated by the time smoke reaches the lower airway. If the lower airway is affected, it may experience trauma in the form of pneumonitis, pulmonary edema, pneumonia, and pulmonary fibrosis (Epstein 2019).
The vascular system experiences a significant loss of integrity when an animal suffers from serious burn injury, “burns frequently cause shock and multiple organ failure because of fluid loss, fluid shifts, electrolyte imbalances, protein losses, myocardial depression, increased peripheral vascular resistance, and increased blood viscosity” (Hedlund 2007). After an area of the body is burned, fluid is pulled from the vascular space into the site of trauma, and subsequently lost from the body (Pashmakova 2016). The normal wound healing process of the body relies heavily on plasma and protein to help to prevent further fluid loss. On top of whole blood and tissue loss, there are the inflammatory processes that an animal is experiencing not only from the burns but also from smoke inhalation injury as well. Overall, these compounding elements emphasize the need for replenishing lost fluid and protein through appropriate fluid therapy and nutritional management.
Burn classifications
Once admitting an animal who has suffered burn trauma, both classifying the severity of the burns and understanding the stages of the healing process are vital to appropriate patient management. It should be taken into consideration that as the biology of a species varies, so can the presentation and classification of burns. In that same respect, the ability for an animal to thrive after surviving burn injury can be contingent upon compromised anatomic function specific to its species, as elements such as scarring near joints, nail loss, etc. can permanently disable an animal’s ability to perform basic necessary functions (Fowler 2020). The incipient classification, first degree burns (Fig. 6, circled portion), is defined as superficial and involves the epidermis only. They are often equated to sun burns. First degree burns may appear as dry, flaky, or erythematous and usually take three to six days to reach re-epithelialization (Hedlund 2007).
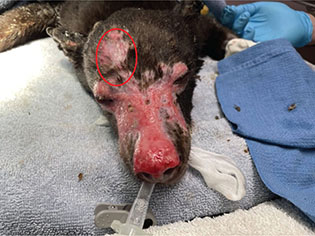
Fig. 6 The circled portion of this image is an example of first degree burns, the remainder of the image shows second degree burns on bear ‘A’.
Second degree burns (Fig. 6, rostrum/periocular space) are partial to intermediate thickness, meaning that they involve the first two layers of skin: the epidermis and the dermis. Second degree burns may appear as moist and erythematous and, depending on the species, may present with blistering (Pashmakova 2016). Generally, second degree burns take one to two weeks to reach re-epithelialization (Pashmakova 2016). Third degree burns (Fig. 7) are considered full thickness, meaning they are deep into the dermis and either close to or involving the adipose layer. Third degree burns appear as red-waxy or white and may have reduced sensory and pain input or eschar formation (Pashmakova 2016).
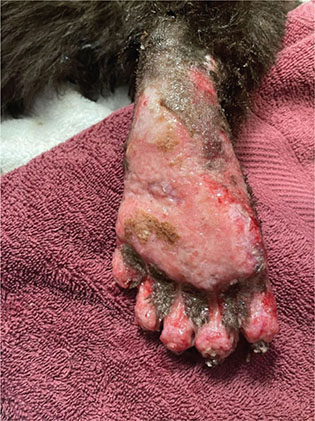
Fig. 7 Third degree burns of the plantar surface of the right hindlimb bear ‘D’. Nail loss can be appreciated in this image as well.
The deeper the burn is, specifically third or fourth degree, the more potential there is for nerve damage, which consequently can affect reception of pain (Epstein 2019). Third degree burns can take two to three weeks to reach re-epithelialization and often require surgical intervention (Pashmakova 2016). Finally, fourth degree burns (Figs. 8 and 9) are deep enough that they involve muscle or bone, appear as pearl white or eschars, and again require surgical intervention to repair (Pashmakova 2016). Fourth degree burns often result in the loss of some function in the affected tissues and require months to heal; digit amputation may even be necessary. Below are some examples of burns of varying degrees, including an example of eschar formation (Fig. 8, circled portion) and ligament exposure (Fig. 9).
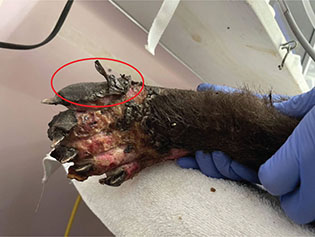
Fig. 8 The circled portion of this image is an example of an eschar; the remainder of the image shows fourth degree burns on the dorsal surface of the right hindlimb of bear ‘D’.
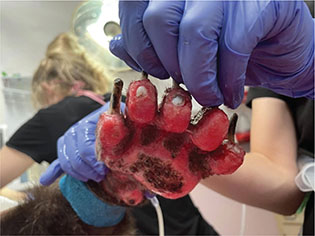
Fig. 9 Fourth degree burns with partial ligament exposure on the palmar surface of the left forelimb of bear ‘D’.
Conclusion
Utilizing burn and smoke inhalation management strategies from small animal medicine was an effective means of appropriate patient management in the treatment of three American black bears rescued from a wildfire. Surviving a wildfire is a significant physiologically traumatic experience for an animal as major body functions are challenged or even compromised from such an event. The first few days of patient management post-wildfire exposure are the most critical with regard to the severity of trauma establishing itself. Early intervention through use of oxygen therapy, wound management, nutrition management, and fluid therapy can be critical in reducing the negative physiological effects of surviving a wildfire. Although the rehabilitation of these patients using the veterinary medical management methods described in this paper was effective in treating three of four cases to release, more information concerning the management of other wildlife species affected by wildfires is needed.
Acknowledgments
The author would like to acknowledge the hard work of the PAWS Wildlife Center staff in rehabilitating the black bears whose admittance prompted this publication, the board-certified veterinary specialists who contributed their knowledge to help in providing high-quality veterinary care for these animals, and WDFW. The author would specifically like to thank Dr Nicki Rosenhagen, Dr Bethany Groves, and Dr John Winter for their direct support in the writing of this paper. The author would additionally like to thank Jeff Brown for the photographic documentation of these patients.
References
| Butkus C.E., Peyton J.L., Heeren A.J. & Clifford D.L. 2021. Prevalence, treatment, and survival of burned wildlife presenting to rehabilitation facilities from 2015 to 2018. Journal of Zoo and Wildlife Medicine 52(2), doi: 10.1638/2020-0093. |
| Carpenter D.H., Macintire D.K. & Tyler J.W. 2001. Acute lung injury and acute respiratory distress syndrome. Vetfolio, August. Accessed on the internet at https://www.vetfolio.com/learn/article/acute-lung-injury-and-acute-respiratory-distress-syndrome on 2 September 2022 |
| Epstein S. 2019. VIN/VECCS rounds: small animal fire-related injuries (public). VIN, 14 January. Accessed on the internet at https://www.vin.com/apputil/project/defaultadv1.aspx?pId=24938&catId=142076&id=9451020 on 2 September 2022 |
| Fowler A. 2020. Treating burnt wildlife. AWRC. Accessed on the internet at https://www.awrc.org.au/uploads/5/8/6/6/5866843/dr_anne_fowler-treating_burns_in_wildlife.pdf on 2 September 2022 |
| Garzotto C.K. 2014. Chapter 140: thermal burn injury. In D. Silverstein, K. Hopper (eds.): Small animal critical care medicine. 2nd ed. Pp. 743–747. St. Louis, MO: Elsevier. |
| Hedlund C.S. 2007. Chapter 15: surgery of the integumentary system. In T.W. Fossum (ed.): Small animal surgery. 3rd ed. Pp. 167, 168, 228, 229. St. Louis, MO: Elsevier Mosby. |
| Jasani S. 2014. Chapter 147: smoke inhalation. In D. Silverstein, K. Hopper (eds.): Small animal critical care medicine. 2nd ed. Pp. 785–788. St. Louis, MO: Elsevier. |
| Maxwell E.A. & Bennett A. 2020. Chapter 11: wound management in wildlife. In S.M. Hernandez, H.W. Barron, E.A. Miller, R.F. Aguilar, M.J. Yabsley (eds.): Medical management of wildlife species: a guide for practitioners. pp. 129–132. Hoboken, NJ: Wiley-Blackwell. |
| Pashmakova M. 2016. Burn patients: the first hour, first day, first week. Vetfolio. Accessed on the internet at https://www.vetfolio.com/courses/burn-patients-the-first-hour-first-day-first-week on 2 September 2022 |
| Sharpe A.N., Gunther-Harrington C.T., Epstein S.E., Li R.H.L. & Stern J.A. 2020. Cats with thermal burn injuries from California wildfires show echocardiographic evidence of myocardial thickening and intracardiac thrombi. Nature News, 14 February. Accessed on the internet at https://www.nature.com/articles/s41598-020-59497-z#:~:text=Echocardiograms%20of%20affected%20cats%20revealed,to%20a%20possible%20cardiac%20cause on 2 September 2022 |
| Terrill J.B., Montgomery R.R. & Reinhardt C.F. 1978. Toxic gases from fires. Science 200(4348), 1343–1347, doi: 10.1126/science.208143. |
| Wildfire Division. 2020. Wildfire season 2020. Washington State Department of Natural Resources. Accessed on the internet at https://www.dnr.wa.gov/publications/rp_fire_annual_report_2020.pdf on 2 September 2022 |