ARTICLE
Evaluating the efficacy of 16 surfactants for removing petrochemicals from feathers
Erica A. Miller1 & Allison Ricko2
1Wildlife Futures Program, Wildlife Medicine, University of Pennsylvania School of Veterinary Medicine; Kennett Square, PA, USA
2Knoell USA, LLC; Garnett Valley, PA, US
Abstract
New detergents are developed, and existing products are reformulated on a regular basis. To ensure that the most effective products are used for decontaminating oiled wildlife, periodic assessment is necessary. Sixteen surfactants previously determined (Ambrose & Tegtmeier 2015) to be subjectively effective at removing oil from feathers (based on appearance and water repellency of the feather) were selected for this objective evaluation. This study used the methods developed and described in previous studies (Bryndza et al. 1991; Miller et al. 2003) to assess these 16 products. Standard quantities of feathers were uniformly oiled with a synthetic oil containing components found in many petroleum spills, then subjected to a “wash,” and rinse process with 1, 2, and 3% dilutions of each of the 16 products. The residue remaining on the washed feather samples was extracted with solvents and analyzed by gas chromatography to determine the quantities of each component present. The resulting data provide a measure of efficacy of each surfactant, allowing for recommendations regarding product use for cleaning oiled birds.
BIO
Erica Miller worked full time as a wildlife rehabilitation veterinarian for 25 years. She is the Field Operations Manager at the Wildlife Futures Program and an Adjunct Associate Professor of Wildlife Medicine at the University of Pennsylvania School of Veterinary Medicine. She volunteers at Mercer County Wildlife Center and Tri-State Bird Rescue & Research. erica@jfrink.com
Allison Ricko was the Scientist and Laboratory Coordinator at Knoell USA, LLC at the time of this study.
ARicko@knoellusa.com
Keywords
Surfactant; petrochemical; oiled wildlife
Abbreviations
GC: gas chromatography
RSD: relative standard deviation
Citation: Wildlife Rehabilitation Bulletin 2022, 39(1), 1–22, http://dx.doi.org/10.53607/wrb.v39.244
Copyright: Wildlife Rehabilitation Bulletin 2022. © 2022 E.A. Miller & A. Ricko. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Accepted: 15 December 2021; Published: 31 May 2022
Competing interests and funding: The author reports no conflict of interest.
This project was supported by the California Department of Fish and Wildlife’s Oil Spill Prevention and Administration Fund through the Oiled Wildlife Care Network at the Karen C. Drayer Wildlife Health Center, School of Veterinary Medicine, University of California, Davis.
Correspondence to: Erica A. Miller, DVM, 1250 Corner Ketch Road, Newark, DE 19711. Erica@JFrink.com
Introduction
In order to restore insulating capabilities and water repellency to oil-contaminated feathers, the feathers must be completely freed of both oil and cleaning agents (Dein & Frink 1986; Miller & Welte 1999). The most important considerations for thorough cleaning are the effectiveness of a given product at removing petroleum at physiological temperatures and the ease of rinsing away the cleaning product (Frink & Miller 1995). Other considerations for the practical use of a surfactant include commercial availability, potential toxicity to the species being washed, cost, and the logistics of supply and handling (Welte et al. 1991; Bryndza et al. 1995).
In 1990, Bryndza et al. developed an objective method of evaluating surfactant efficacy for removing petrochemicals from contaminated feathers (Bryndza et al. 1991). The results of that study, as well as similar studies conducted on cleaning products in 1995, 2003, and 2006, demonstrated that Dawn® dishwashing liquid detergent (Procter & Gamble, Cincinnati, OH) was more effective than other agents at removing a synthetic oil from uniformly oiled feathers in a laboratory situation (Bryndza et al. 1991; Bryndza et al. 1995; Miller et al. 2003; Miller et al. 2006).
Subjective evaluation of 25 new products (Ambrose & Tegtmeier 2015) was used to select the products chosen for this objective testing.
Materials and methods
Sixteen cleaning products were selected for evaluation based on prior objective testing and the results of the subjective testing by Ambrose & Tegtmeier (2015) (Appendix A, Table 1). The subjective testing was completed approximately 3 years prior to this trial; consequently, not all the same products were available, and some formulations may have changed. Bear Paw™ Nature Cleanse was no longer available, so the product Bear Paw™ Hand Cleaner was used in the objective testing. The CitraSolv™ CitraDish® Natural Dish Soap used in the subjective testing is now sold under the brand HomeSolv™ CitraDish® Natural Dish Soap (but is advertised to be the same product). Mixed Chicks® “detangling” shampoo was tested by Ambrose and Tegtmeier; this formulation is no longer available so Mixed Chicks® “clarifying” shampoo was used for this study. All other products were the same name and manufacturer as those used in the subjective testing; indeed, many were the same bottles. It is possible, though unlikely, that some components may have denatured in the period between the two trials.
All products were placed into uniform bottles and assigned an identification letter (A–P) to eliminate potential bias on the part of the investigator (Ricko). The final list of all products tested is presented in Appendix A, Table 2.
Objective testing
To conduct an objective evaluation of these products, the method described by Bryndza et al. (Bryndza et al. 1991; Bryndza et al. 1995) was used, with some procedural changes resulting from improved technology and more accurate laboratory equipment. For the method to be reproducible, a mixture of commonly available hydrocarbons was made to serve as the contaminating “oil.” This synthetic oil contained equal amounts (by mass) of 13 components representing the types of molecules found in light petroleum mixtures such as kerosene, mineral oil, diesel fuel, home heating oil, and light crude oil. The same types of chemical structures and functional groups are present in heavy crude oils and tars as well, making this mixture versatile enough to appropriately represent a wide range of petroleum fractions (Bryndza et al. 1991).
The feathers were oiled by dissolving the synthetic hydrocarbon mixture in a volatile solvent (methylene chloride) and allowing the feathers to stand in the mixture (as described in Appendix B, “Oiling of Feathers”). The methylene chloride acted as a carrier for the hydrocarbon mixture, creating a true homogeneous solution that was able to contact all feather surfaces, thus providing a more uniform oiling of the feathers. After standing for an hour, the excess oil was drained, and the last traces of the volatile solvent were removed under vacuum at room temperature.
Experiments were conducted to determine the consistency of oiling by this method. The oil was extracted from the feathers by treating them with acetone and then with a methylene chloride solution containing 1 mg/mL 1-octadecene, as described by Bryndza et al. (1991). The decanted solution was evaluated by GC, allowing the components to be measured as the weight of oil/weight of oiled feathers. This procedure was executed eight times to demonstrate that the oiling of the feather samples was uniform.
The remaining feather samples were then “cleaned” using a reproducible wash/rinse/extraction procedure to determine the effectiveness of each of the cleaning products at removing the deposited compounds (see Appendix B, “Testing of Cleaning Agents”). The oiled feather samples were initially shaken with cleaning solutions and then with two water rinses to simulate the subjective clinical process of washing and rinsing oiled birds in a reproducible manner. All cleaning products, feather samples, and water rinses were maintained at 40°C (104°F), as this temperature approximates avian body temperature and has been shown to be effective in cleaning birds by standard protocols. The 16 cleaning products were evaluated in this manner at three different concentrations (3, 2, and 1%). Local tap water (hardness = ca. 3 grains/gallon or 0.05 ppm) was used to prepare the solutions and to rinse the feathers after washing.
After the rinse, the feather samples were extracted first with acetone (to remove water and some residual oil) and then with a solution of methylene chloride containing a known amount of the nonvolatile internal standard 1-octadecene (used to analyze the amount of each component present on the feathers after washing and rinsing).
The combined acetone and methylene chloride extracts were dried with anhydrous magnesium sulfate (MgSO4) (to remove water from the extraction) and then filtered to remove the MgSO4. The amounts of the individual components present in a filtered solution of oil residue and 1-octadecene were determined by quantitative GC.
A control was provided for each of the three sets by conducting the process on three feather samples without the addition of a cleaning agent (10 mL of water was added in place of the 10 mL cleaning solution).
Results
Uniformity of oiling
As seen in Table 1 and illustrated in Fig. 1, the RSD of the components revealed the feathers to be oiled within approximately ±12.5% of a mean value for 12 components (the ethylcyclohexane was found too volatile to reproducibly quantify). This was less uniform than previous studies, which were all within 10% of a mean value (Bryndza et al. 1991; Bryndza et al. 1995; Miller et al. 2003; Miller et al. 2006).
| Uniformity of Oiling | |||||||||||
| Component | Measured Sample Concentration (mg/mL) | SD | RSD (%) | ||||||||
| UC-1 | UC-2 | UC-3 | UC-4 | UC-5 | UC-6 | UC-7 | UC-8 | Average | |||
| 2-Ethylnaphthalene | 184 | 67.7 | 61.9 | 78.4 | 104 | 76.5 | 133 | 112 | 102 | 41.0 | 40 |
| 2-Methylheptane | 201 | 208 | 268 | 175 | 233 | 264 | 194 | 275 | 227 | 38.2 | 17 |
| cis-Decalin | 134 | 140 | 134 | 136 | 104 | 147 | 119 | 105 | 127 | 16.1 | 13 |
| Ethylcyclohexane | 314 | NAa | ND | ND | ND | ND | ND | ND | 314 | NAb | NA |
| Mesitylene | 60.2 | 55.8 | 57.8 | 56.4 | 55.3 | 64.4 | 56.9 | 61.1 | 58.5 | 3.15 | 5.4 |
| n -Butybenzene | 59.4 | 61.1 | 53.6 | 57.2 | 51.2 | 60.2 | 56.4 | 53.9 | 56.6 | 3.52 | 6.2 |
| n -Butylcyclohexane | 53.1 | 48.1 | 47.9 | 47.0 | 46.8 | 49.5 | 49.2 | 52.3 | 49.2 | 2.34 | 4.8 |
| n -Dodecane | 65.2 | 66.0 | 63.3 | 65.2 | 24.9 | 67.4 | 57.2 | 52.3 | 57.7 | 14.2 | 25 |
| n -Eicosane | 94.9 | 42.6 | 40.2 | 43.2 | 61.1 | 37.1 | 60.8 | 55.3 | 54.4 | 18.9 | 35 |
| Naphthalene | 207 | 173 | 188 | 193 | 165 | 195 | 197 | 186 | 188 | 13.5 | 7.2 |
| o -Xylene | 52.8 | 54.2 | 62.2 | 53.4 | 56.7 | 71.0 | 51.2 | 61.1 | 57.8 | 6.62 | 11 |
| p-Cresol | 23.9 | 23.4 | 28.9 | 23.9 | 17.1 | 26.8 | 19.8 | 20.7 | 23.1 | 3.80 | 16 |
| Tetralin | 759 | 974 | 877 | 921 | 564 | 850 | 578 | 666 | 774 | 157 | 20 |
| TOTAL | 2209 | 1914 | 1883 | 1850 | 1483 | 1909 | 1573 | 1701 | 1815 | 227 | 12.5 |
| a ND = no peak detected b NA = not applicable |
|||||||||||
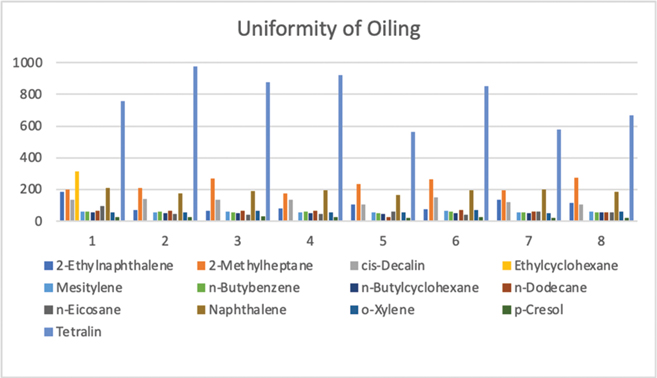
Fig. 1 Illustration of the sample concentration (mg/mL) of oil components on eight randomly selected samples of oiled feathers (Evaluation of Uniformity of Oiling).
Controls
The three control washes produced inconsistent results (Table 2 and Fig. 2). Total residues ranged from 1716 to 11 546 mg/mL. Ratios of the components were generally consistent between controls #2 and #3, but the quantities varied considerably. Values of components in control #3 were 1.5 to 2 times the values of components in control #2, with the exception of naphthalene and ethylcyclohexane. Naphthalene in control #3 was 3.5 times that of control #2, and ethylcyclohexane was 562 mg/mL in control #3, but not detected in control #2. Neither was ethylcyclohexane detected in control #1, nor was any n-Eicosane detected in control #1. Furthermore, the values of the components in control #1 varied from 3.5 to 15 times those of control #2.
| Component | Measured Sample Concentration (mg/mL) | ||
| Control #1 | Control #2 | Control #3 | |
| 2-Ethylnaphthalene | 364 | 104 | 204 |
| 2-Methylheptane | 1251 | 287 | 513 |
| cis-Decalin | 863 | 145 | 249 |
| Ethylcyclohexane | NDa | ND | 562 |
| Mesitylene | 401 | 65.1 | 108 |
| n-Butybenzene | 389 | 63.4 | 95.7 |
| n-Butylcyclohexane | 331 | 52.1 | 94.8 |
| n-Dodecane | 352 | 66.9 | 128 |
| n-Eicosane | ND | 48.9 | 67.9 |
| Naphthalene | 1734 | 151 | 359 |
| o-Xylene | 380 | 53.3 | 115 |
| p-Cresol | 293 | 19.2 | 33.4 |
| Tetralin | 5187 | 659 | 1349 |
| TOTAL | 11546 | 1716 | 3878 |
| a ND = no peak detected | |||
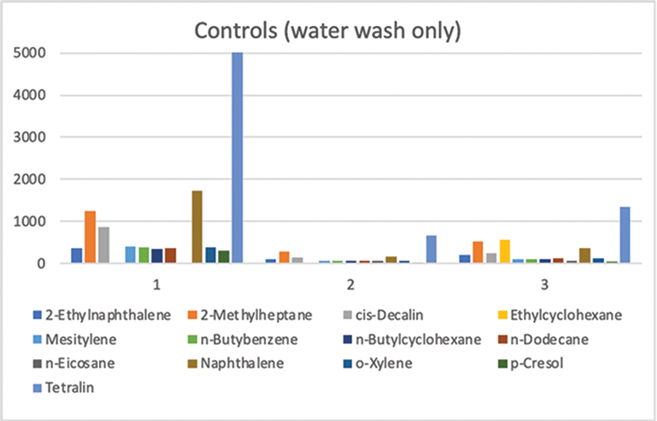
Fig. 2 Illustration of the amount (mg/mL) of each synthetic oil component remaining on the feathers after the three control washes (water only, no detergent).
Efficacy of cleaning agents
A summary of the GC analysis showing the relative amounts of each component (in mg) remaining on the 2.0 g samples of oiled feathers after cleaning is shown in Tables 3–5. The control data reported represent the amounts of contaminants remaining on feather samples after three washes with water alone, that is, in the absence of detergents. Tables 3–5, respectively, report results obtained using 3, 2, and 1% v/v solutions of detergents for the wash step. Table 6 summarizes the total weight of contaminants remaining on feather samples (the sums of the columns in Tables 3–5) after washing and rinsing as a function of the cleaning agent and concentration. While this is a simplistic method that does not attempt to correlate chemical structure with ease of removal, it does give a single numerical evaluation to a given washing protocol. Based on these numerical values, detergent H (Palmolive® Ultra Strength™) left the least amount of oil after washes with each of the three dilutions (Table 6).
| 1% Cleaning Solutions | |||||||||||||||||
| Component | Measured Sample Concentration (mg/mL) | ||||||||||||||||
| Control | 1% A | 1% B | 1% C | 1% D | 1% E | 1% F | 1% G | 1% H | 1% I | 1% J | 1% K | 1% L | 1% M | 1% N | 1% O | 1% P | |
| 2-Ethylnaphthalene | 364 | 47.8 | 54.2 | 47.2 | 63.9 | 92.8 | 91.3 | 84.9 | 33.7 | 106 | 126 | 134 | 75.4 | 66.0 | 83.1 | 87.2 | 45.4 |
| 2-Methylheptane | 1251 | 131 | 199 | 100 | 283 | 301 | 510 | 187 | 35.7 | 355 | 326 | ND | 0.224 | 236 | 159 | 144 | ND |
| cis-Decalin | 863 | 57.0 | 105 | 53.1 | 178 | 159 | 277 | 28.3 | 26.2 | 172 | 140 | 390 | 109 | 1827 | 58.4 | 70.8 | 16.0 |
| Ethylcyclohexane | NDa | ND | ND | ND | ND | ND | ND | ND | ND | ND | 184 | ND | ND | ND | ND | ND | ND |
| Mesitylene | 401 | 27.1 | 52.0 | 22.2 | 61.5 | 59.1 | 141 | 14.2 | 11.7 | 67.3 | 61.6 | 51.2 | 52.4 | 79.0 | 25.6 | 20.9 | 9.20 |
| n-Butybenzene | 389 | 2.74 | 50.1 | 23.4 | 32.5 | 61.7 | 18.9 | 93.9 | 11.9 | 73.8 | 67.7 | 2.11 | 50.9 | 78.2 | 55.7 | 32.9 | 15.0 |
| n-Butylcyclohexane | 331 | 24.3 | 43.4 | 2.03 | 59.8 | 42.4 | 117 | 25.3 | 9.67 | 59.7 | 58.6 | 38.6 | 1.60 | 68.3 | 28.7 | 35.8 | 13.4 |
| n-Dodecane | 352 | 24.2 | 52.0 | 23.0 | 72.2 | 74.9 | 115 | 1.60 | 13.1 | 77.8 | 68.0 | 75.2 | 11.9 | 92.2 | 39.2 | 35.1 | 1.25 |
| n-Eicosane | ND | 21.4 | 29.5 | 19.2 | 37.6 | 40.4 | 40.1 | 41.3 | 11.1 | 58.3 | 57.9 | 66.6 | 45.1 | 36.6 | 49.5 | 36.4 | 16.8 |
| Naphthalene | 1734 | 116 | 154 | 106 | 153 | 177 | 209 | 165 | 67.9 | 184 | 150 | 170 | 165 | 176 | 193 | 121 | 101 |
| o-Xylene | 380 | 28.7 | 47.1 | 21.2 | 70.3 | 59.4 | 132 | 2.64 | 10.2 | 77.6 | 68.5 | 174 | 16.2 | 74.6 | 35.2 | 18.6 | 5.02 |
| p-Cresol | 293 | 18.8 | 22.5 | 21.3 | 22.0 | 18.0 | 51.0 | 28.2 | 18.4 | 21.3 | 15.0 | 4.76 | 24.8 | 17.7 | 29.5 | 24.5 | 25.8 |
| Tetralin | 5187 | 401 | 556 | 374 | 478 | 1030 | 112 | 334 | 176 | 966 | 664 | ND | 524 | 620 | 806 | 649 | 244 |
| TOTAL | 11546 | 900 | 1365 | 813 | 1512 | 2116 | 1815 | 1007 | 426 | 2218 | 1987 | 1106 | 1076 | 3372 | 1564 | 1277 | 493 |
| a ND = no peak detected | 4 | 9 | 3 | 10 | 14 | 12 | 5 | 1 | 15 | 13 | 7 | 6 | 16 | 11 | 8 | 2 | |
| 2% Cleaning Solutions | |||||||||||||||||
| Component | Measured Sample Concentration (mg/mL) | ||||||||||||||||
| Control | 2% A | 2% B | 2% C | 2% D | 2% E | 2% F | 2% G | 2% H | 2% I | 2% J | 2% K | 2% L | 2% M | 2% N | 2% O | 2% P | |
| 2-Ethylnaphthalene | 104 | 18.3 | 30.7 | 24.6 | 49.8 | 46.7 | 69.1 | 66.4 | 16.2 | 91.7 | 98.7 | 57.9 | 61.5 | 33.2 | 131 | NDa | NAb |
| 2-Methylheptane | 287 | 24.1 | 38.7 | 27.6 | 69.6 | 68.3 | 93.1 | 106 | 24.1 | 299 | 348 | 149 | 133 | 67.5 | 357 | ND | NA |
| cis-Decalin | 145 | 14.4 | 23.0 | 18.9 | 41.8 | 37.2 | 63.7 | 51.2 | 14.6 | 104 | 132 | 59.0 | 57.6 | 31.4 | 148 | ND | NA |
| Ethylcyclohexane | ND | ND | ND | ND | ND | ND | 412 | ND | ND | ND | ND | ND | ND | ND | ND | ND | NA |
| Mesitylene | 65.1 | 7.02 | 10.8 | 8.69 | 18.6 | 17.6 | 29.9 | 23.9 | 6.48 | 51.8 | 61.7 | 29.7 | 29.6 | 15.4 | 77.2 | 3.70 | NA |
| n-Butybenzene | 63.4 | 6.68 | 10.2 | 8.41 | 19.6 | 17.0 | 30.9 | 25.0 | 6.32 | 50.1 | 60.2 | 27.4 | 28.2 | 13.9 | 42.9 | 43.4 | NA |
| n-Butylcyclohexane | 52.1 | 5.42 | 8.39 | 6.83 | 16.2 | 13.2 | 23.7 | 20.4 | 5.53 | 45.8 | 48.9 | 23.2 | 23.1 | 11.9 | 77.1 | ND | NA |
| n-Dodecane | 66.9 | 6.25 | 10.4 | 9.03 | 21.1 | 16.1 | 28.1 | 26.7 | 7.26 | 48.7 | 58.9 | 23.8 | 26.5 | 12.9 | 72.4 | ND | NA |
| n-Eicosane | 48.9 | 5.71 | 8.52 | 7.69 | 15.9 | 12.8 | 23.8 | 20.5 | 6.72 | 33.6 | 45.3 | 18.1 | 18.3 | 12.5 | 57.9 | ND | NA |
| Naphthalene | 151 | 36.2 | 50.5 | 42.9 | 110 | 85.6 | 132 | 121 | 32.4 | 165 | 134 | 127 | 133 | 63.3 | 147 | 8.67 | NA |
| o-Xylene | 53.3 | 6.99 | 10.3 | 8.00 | 16.6 | 16.5 | 26.2 | 23.2 | 6.02 | 56.0 | 63.7 | 31.8 | 30.0 | 15.1 | 90.0 | ND | NA |
| p-Cresol | 19.2 | 17.9 | 16.5 | 16.0 | 17.1 | 13.5 | 13.8 | 18.3 | 18.1 | 20.8 | 13.9 | 17.6 | 23.6 | 18.6 | 12.9 | 16.7 | NA |
| Tetralin | 659 | 99.2 | 173 | 143 | 349 | 306 | 511 | 451 | 96.3 | 547 | 570 | 489 | 446 | 233 | 545 | 512 | NA |
| TOTAL | 1716 | 248 | 391 | 322 | 746 | 650 | 1458 | 954 | 240 | 1513 | 1636 | 1054 | 1011 | 529 | 1759 | 584 | NA |
| a ND = no peak detected | 2 | 4 | 3 | 8 | 7 | 12 | 9 | 1 | 13 | 14 | 11 | 10 | 5 | 15 | 16 | ||
| b NA = not applicable; due to an unknown error, no internal standard was present in Sample P and the data will not be reported | |||||||||||||||||
| 3% Cleaning Solutions | |||||||||||||||||
| Component | Measured Sample Concentration (mg/mL) | ||||||||||||||||
| Control | 3% A | 3% B | 3% C | 3% D | 3% E | 3% F | 3% G | 3% H | 3% I | 3% J | 3% K | 3% L | 3% M | 3% N | 3% O | 3% P | |
| 2-Ethylnaphthalene | 204 | 18.2 | 24.2 | 15.4 | 27.0 | 20.4 | 29.6 | 20.9 | 12.9 | 111 | 54.2 | 30.0 | 42.8 | 21.5 | 118 | 27.8 | 18.9 |
| 2-Methylheptane | 513 | 19.3 | 45.0 | 32.2 | 44.6 | 40.6 | 43.5 | 41.8 | 26.0 | 255 | 243 | NDa | ND | 19.0 | 191 | 41.5 | 40.6 |
| cis-Decalin | 249 | 12.6 | 21.0 | 15.3 | 21.3 | 17.0 | 24.3 | 18.8 | 11.6 | 102 | 97.9 | 2.49 | 42.6 | 15.1 | 94.4 | 23.8 | 18.2 |
| Ethylcyclohexane | 562 | ND | 413 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Mesitylene | 108 | 6.00 | 10.0 | 7.09 | 10.1 | 8.33 | 11.7 | 9.24 | 5.40 | 41.2 | 46.0 | 16.1 | 21.6 | 6.67 | 38.4 | 11.5 | 9.21 |
| n-Butybenzene | 95.7 | 5.98 | 9.73 | 6.57 | 9.57 | 7.65 | 11.0 | 8.41 | 5.14 | 42.1 | 39.2 | ND | 20.0 | 6.71 | 42.1 | 11.2 | 8.69 |
| n-Butylcyclohexane | 94.8 | 4.70 | 7.75 | 6.05 | 8.07 | 6.54 | 9.31 | 7.31 | 4.60 | 36.7 | 39.9 | ND | 4.02 | 5.49 | 32.5 | 8.87 | 6.76 |
| n-Dodecane | 128 | 6.36 | 10.2 | 7.01 | 10.6 | 7.37 | 12.7 | 8.15 | 5.56 | 51.7 | 45.5 | 0.0323 | 16.3 | 7.84 | 41.0 | 11.7 | 7.38 |
| n-Eicosane | 67.9 | 6.94 | 8.13 | 8.46 | 8.36 | 6.64 | 11.3 | 7.44 | 5.40 | 44.9 | 42.4 | 9.64 | 15.1 | 8.52 | 34.9 | 8.15 | 6.13 |
| Naphthalene | 359 | 36.2 | 55.7 | 34.5 | 53.0 | 42.4 | 52.8 | 42.5 | 25.1 | 145 | 134 | 76.2 | 105 | 41.0 | 141 | 73.9 | 47.7 |
| o-Xylene | 115 | 5.55 | 9.71 | 7.16 | 10.1 | 8.39 | 12.1 | 9.34 | 5.68 | 42.0 | 46.3 | 16.4 | 14.9 | 5.78 | 38.5 | 10.6 | 9.36 |
| p-Cresol | 33.4 | 18.3 | 22.9 | 20.4 | 20.8 | 18.1 | 12.2 | 15.6 | 19.7 | 34.3 | 27.9 | 24.5 | 31.2 | 16.0 | 26.8 | 24.0 | 25.4 |
| Tetralin | 1349 | 89.9 | 137 | 91.4 | 152 | 112 | 161 | 111 | 70.4 | 620 | 530 | 188 | 294 | 98.6 | 567 | 162 | 117 |
| TOTAL | 3878 | 230 | 774 | 251 | 375 | 295 | 391 | 301 | 197 | 1525 | 1347 | 364 | 607 | 252 | 1366 | 415 | 315 |
| a ND = no peak detected | 2 | 13 | 3 | 9 | 5 | 10 | 6 | 1 | 16 | 14 | 8 | 12 | 4 | 15 | 11 | 7 | |
Discussion
Uniformity of oiling
The differences in oil distribution are most likely due to variation in feathers—for example, those with broken shafts would have trapped more oil inside the shafts than those with intact shafts. It is also possible that the feathers experienced more clumping as a different type of container was used for this study than in previous studies. Ethylcyclohexane was only found in the first sample; this volatile compound most likely dissipated from the other samples (Table 1 and Fig. 1). This variation suggests that there may have been sufficiently different amounts of oil on the feather samples to affect the outcome of the washing trials or possibly that the procedure was not conducted in exactly the same manner each time.
Controls
The results from the three controls run varied greatly from the expected consistent values obtained in past studies. The differences in total amounts of oil remaining on the feathers could be explained by nonuniformity in oiling of the feathers; however, the variation in the components of each oil remaining on each sample cannot be explained. Inconsistency in sample handling or treatment may have occurred between the controls, as well as between each sample, potentially invalidating all results. The investigators can provide no explanation for n-Eicosane and ethylcyclohexane appearing in at least one control but not in the others, except for possible variation in treatment of the samples (e.g., longer exposure to air allowing for more evaporation of these volatile components).
Efficacy of cleaning agents
The results in Table 6 and Fig. 3 show dramatic differences in oil removal among the cleaning agents tested. The four products that were consistently the most effective in removing the oil were H, P, A, and C (Palmolive® Ultra Strength™, Citrus Fresh Dish Soap, Dawn® Ultra Dishwashing Liquid Original Scent, and Fairy Liquid Original, respectively). Of these, only the Dawn® Ultra was ranked in the top four on the subjective feather testing, most likely due to differences in the oils used in the subjective (light crude oil) vs objective (synthetic oil) trials. The other three top-ranking detergents from the subjective feather testing ranked 8th, 9th, and 13th in the objective testing (products B, D, and M in Fig. 3), suggesting these products may be more effective at removing light crude oil than the synthetic oil. Excluding the 1% run for product M (HomeSolv™ CitraDish®), this product did very well on both the subjective and objective testing; future testing should include further objective testing of this product.
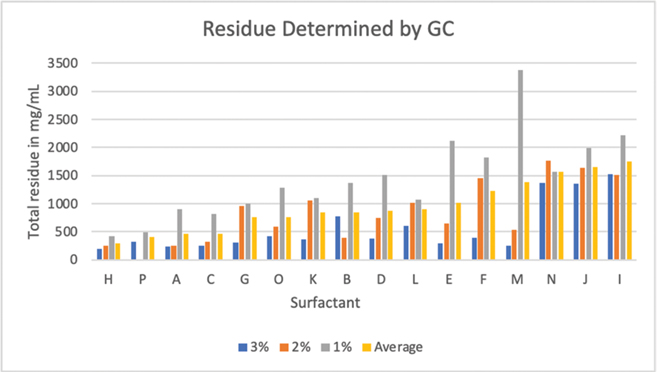
Fig. 3 Illustration of the total amount (mg/mL) of synthetic remaining on the feathers after the 1, 2, and 3% washes with the 16 detergents, A–P.
A laboratory error occurred during the 2% run for product P (Citrus Fresh Dish Soap) so the efficacy of this run was not determined. While the product was very effective in the 1 and 3% runs, it cannot be considered for future testing as manufacturing has been discontinued and the product is no longer available.
Further examination of Table 6 and Fig. 3 demonstrate, as expected, most of the products removed more oil when the cleaning agent was used at higher concentrations. Product N (Bitu-Ox™), however, performed worse at the 2% concentration than at either 1 or 3%. This was likely due to nonuniformity in oiling of the feather samples or other experimental error. At the concentrations tested, Bitu-Ox™ failed to remove oil as effectively as 13 of the other products, regardless of the concentration. Similarly, product B (Joy® Ultra Lemon Dish Soap), performed worse at 3% concentration than at either 1 or 2%. This again may have been an experimental error and should be repeated in future testing. If the error is actually in the 3% run rather than the 2% run, this product has good potential as an effective surfactant for cleaning feathers.
Tables 3–5 show that five cleaning products, I, J, L, M, and N (Amodex® Stain Remover, Renew All Purpose Cleaner, Bear Paw™ Hand Cleaner, HomeSolv™ CitraDish®, and Bitu-Ox™, respectively) left more residues of certain components than the control, that is, they removed less of these oil components than water alone. Most of these higher residues were left when the cleaning products were used at lower concentrations, suggesting the products may have been below critical micelle level (insufficient detergent to surround and remove the oil on a molecular level), resulting in a polarity that repelled the water and trapped the oil on the feathers, thereby preventing the oil from coming off in the rinse. Of these products, only HomeSolv™ CitraDish® functioned well enough at the higher concentrations to be considered for future testing.
Additional subjective trial
Due to the questionable nature of the objective test controls and cleaning results, a blind trial was arranged to subjectively evaluate the performance of Palmolive® Ultra Strength™ vs Dawn® Ultra.
General procedure
Four previously frozen Canada goose (Branta canadensis) carcasses (died or euthanized due to presenting injuries) were thawed and examined to confirm that none had visible feather damage or contamination. The carcasses were each floated for 24 h in one of four tubs containing 4 L of water plus 120 mL HD SAE 30 motor oil to simulate the contamination of a bird swimming in oiled water. Each carcass was then washed by the same team of two experienced washers who were not informed of which detergents they were using (see Appendix C for the method used to wash and rinse the oiled carcasses).
An additional experienced wash person was asked to evaluate the washed carcasses. This evaluator was not informed of which products were used in the testing and was simply asked to examine the carcasses and rank them from most waterproof to least waterproof. The results, shown in Table 7, found that Palmolive® Ultra Strength™ cleaned the carcass more effectively than the other products.
Conclusions
The three “trials”—the subjective testing using a light crude oil, the objective testing using the synthetic oil, and the final carcass wash using a motor oil—all showed that both Dawn® Ultra and Palmolive® Ultra Strength™ are effective at removing the oils from feathers.
While the objective testing appears to be fraught with errors, the Palmolive® Ultra Strength™ consistently left the least residue from the synthetic oil based on the GC results. The single wash test and subjective evaluation were consistent with these findings.
To verify these results, the objective testing will be repeated for the three available top-ranking products (Palmolive® Ultra Strength™, Dawn® Ultra Dishwashing Liquid Original Scent, and Fairy Liquid Original) and the HomeSolv™ CitraDish®. Additional carcass washes using a variety of oils will also be conducted to compare the efficacy of the products on the different contaminants.
Acknowledgments
Thank you to Knoell USA for supplying the laboratory, equipment, chemicals, and technical support; to Andrea Ambrose for the subjective testing; Jean Hedrich for assistance in the washing trials; and to the Oil Department staff at Tri-State Bird Rescue & Research, Inc. for assistance with the product evaluation.
This paper was presented at the International Effects of Oil on Wildlife Conference, May 2018 in Baltimore, Maryland.
References
Ambrose A. & Tegtmeier S. 2015. A subjective evaluation of the efficacy of 32 surfactants in the removal of petrochemicals from feathers. May 2015. Proceedings, 12th International Effects of Oil on Wildlife Conference. Anchorage, AK.
Bryndza H.E., Foster J.P., McCartney J.H., Lober J.C. & Lundberg B. 1995. Methodology for determining surfactant efficacy in removal of petrochemicals from feathers. In C. Rineer-Garber (ed.): Wildlife and oil spills: response, research, and contingency planning. Pp. 69–86. Newark, DE: Tri-State Bird Rescue & Research, Inc.
Bryndza H.E., Foster J.P., McCartney J.H., Lundberg B. & Lober J.C. 1991. Surfactant efficacy in removal of petrochemicals from feathers. In L. Frink, K. Ball-Weir & C. Smith (eds.): The effects of oil on wildlife: research, rehabilitation and general concerns. Pp. 78–94. Suisun, CA: International Wildlife Rehabilitation Council.
Dein F.J. & Frink L.S. 1986. Rehabilitation of oil‑contaminated birds. In R.W. Kirk (ed.): Current veterinary therapy. Vol. IX. Pp. 719–722. Philadelphia, PA: W.B. Saunders.
Frink L. & Miller E.A. 1995. Principles of oiled bird rehabilitation. In C. Rineer-Garber (ed.): Wildlife and oil spills: response, research, and contingency planning. Pp. 61–68. Newark, DE: Tri-State Bird Rescue & Research, Inc.
Miller E., Keller J. & Bryndza H. 2006. An evaluation and comparison of some current products for the removal of petrochemicals from feathers. In K. Evans & R. Dunne (eds.): The effects of oil on wildlife: proceedings of the eighth international conference. Pp. 85–99. Newark, DE: Tri-State Bird Rescue & Research, Inc.
Miller E.A., Bryndza H., Milionis C., Meenan K. & Simmons M. 2003. An evaluation of the efficacy of eighty-six products in the removal of petrochemicals from feathers. The effects of oil on wildlife: proceedings of the sixth international conference. October 2003. Myrtle Beach, SC.
Miller E.A. & Welte S.C. 1999. Caring for oiled birds. In M.E. Fowler & R.E. Miller (eds.): Zoo & wild animal medicine: current therapy 4. Pp. 300–308. W.B. Philadelphia, PA: Saunders Co.
Welte S.C., Bryndza H. & Embick J.R. 1991. Notes on health and safety concerns when handling oil contaminated wildlife. In J. White & L. Frink (eds.): The effects of oil on wildlife: research, rehabilitation and general concerns. Pp. 73–77. Suisun, CA: International Wildlife Rehabilitation Council.
Appendix A
Results of subjective testing and product information
| Surfactants | Avg. Score in Short-term Trial | Avg. Score in Long-term Trial |
| HomeSolv™ CitraDish® | 4.0 | 3.9 |
| Dawn®Ultra | 3.9 | 3.7 |
| Method® | 3.8 | 3.6 |
| Joy®Lemon* | 3.7 | 3.9 |
| Dr. Bonner’s Pure Castile Soap | 3.6 | 3.3 |
| Renew All Purpose Cleaner | 3.5 | 2.6 |
| Palmolive®Ultra Strength™ | 3.4 | 2.6 |
| Seventh Generation™ | 3.3 | 3.3 |
| BioGreen Clean® | 3.2 | 2.8 |
| Bitu-Ox™ | 3.2 | 2.9 |
| Mixed Chicks®Shampoo | 3.2 | 2.5 |
| Charlie’s Soap® | 3.1 | 3.1 |
| Citrus Fresh Dish Soap | 3.0 | 2.8 |
| Amodex®* | 2.8 | 3.3 |
| Fairy Original* | 2.8 | 4.0 |
| Bear Paw™ Nature Cleanse* | 2.3 | 3.1 |
Those with asterisks were more effective at cleaning the feathers with “aged” oil (long-term) than the freshly oiled feathers (short-term).
Appendix B
Objective/quantitative evaluation process
Preparation of feathers
Feathers were collected from the breasts of carcasses of six snow geese1 (Chen caerulescens), none of which displayed any evidence of oil contamination. Approximately 122 g of breast feathers were plucked, carefully separated from the down, and stored in polyethylene bags.
Preparation of oil
The synthetic oil was prepared by mixing 69.3–85.1 mg of each of the 13 components (quantity was based on product purity) in a 2-L glass jar.
Oiling of feathers
One liter of the synthetic hydrocarbon mixture was dissolved in 1 L of methylene chloride. The feathers were added to this solution and mixed for 2 min by shaking the jar. The jar was left to sit for 1 h with occasional shaking. The solution was decanted, and the feathers were pressed onto a vacuum filter. The funnel containing the feathers was covered with a paper towel secured with a rubber band, and the funnel was placed into a Vacuum Atmospheres antechamber for 30 min to remove the last traces of the volatile solvent under dynamic vacuum at room temperature.
The feathers were then removed from the vacuum and placed into sealed bags (used to prevent losses of volatile components) in a covered desiccator. A slight vacuum was drawn, and the feathers were allowed to “age” overnight in the desiccator.
The oiled feathers were weighed into glass jars (2.00 ± 0.1 g in each jar), and the lids were taped shut. A total of 59 sample jars was prepared.
Experiments were conducted to demonstrate the uniformity of the oiling of the feather samples. Eight jars were chosen at random, and the samples were treated with 10 ml acetone (to remove any water), shaken for 60 s, and decanted. The feathers were then treated with 50 mL of a methylene chloride/1-octadecene solution (0.2 mg/mL), shaken for 60 s, and decanted. The methylene chloride was used to extract the oil, and the 1-octadecene acted as an internal standard to quantify the oil components. The feathers were placed on a vacuum filter, and 1 mL of the extracted solution was placed in a GC vial. GC was used to quantify the components of the oil in the extraction relative to the 50 mg/sample internal standard amount of 1-octadecene present.
Testing of cleaning agents
To each jar containing a 2-g sample of oiled feathers, 10 mL of a cleaning solution was added at 40°C (2, 1, or 0.5% solutions). The jar was shaken vigorously for 30 s, and the solution was decanted. Ten milliliters of 40°C water was added to the jar/feathers, shaken vigorously for 30 s, and decanted. A second 10 mL of 40°C water was added to the jar/feathers, shaken vigorously for 30 s, and decanted. Ten mL of acetone were added to the jar/feathers (to remove any water) and shaken vigorously for 60 s, then decanted onto a filter frit containing approximately 2 mg of magnesium sulfate (MgSO4). A final 50 mL of methylene chloride/1-octadecene (0.2 mg/mL) was added to the jar/feathers and shaken vigorously for 60 s and then emptied onto the filter to remove the solids. The jar was rinsed with methylene chloride to remove any remaining oil, and this rinse was poured over the feathers to further extract any residues. A 1-ml sample of the filtered residue was then placed in a vial, capped, and analyzed by GC within 24 h.
Creating a control for the process. For each dilution (1, 2, and 3%), the procedure was repeated on a 17th sample as a control, using 10 mL of water in place of the 10 mL of cleaning solution.
Evaluation. The GC results provided the components of oil residue (in mg) remaining on the feathers as compared to the internal standard (1-octadecene). The GC results for each cleaning product were totaled and entered in Table 6 (illustrated in Fig. 3).
Appendix C
Method for carcass2 wash and evaluation
Washtubs were prepared using 58 ounces of detergent in 15 gallons of water in the first tub (3% solution), 38 ounces of the same detergent in 15 gallons of water in the second tub (2% solution), and 15 gallons of water with no detergent in the third tub. All water used was tap water from the same source, and the water temperature of each tub was 105–106°F (40.5–41°C). Rinse water was also tap water from this same source, at 105–106°F.
The wash team consisted of two experienced individuals who were directed to wash the bird for as long as they felt necessary in each of the first two tubs, moving to the next tub (second or third) when they were ready to do so. They then rinsed the bird in the warm water tub for 1 min before moving to the spray rinse station. After removing their wash gloves and rinsing their arms and aprons, the team commenced rinsing the carcasses and continued until they thought the bird was completely rinsed. The amount of time each bird was kept in each tub and in the rinse was recorded (Table 7).
This process was repeated three times for a total of four washes. Each set of tubs was prepared in the absence of the wash team, so that neither wash person knew which detergent was used.
After all four carcasses were washed, another experienced washer was asked to inspect the cadavers, evaluate them for waterproofing, and then rank them in order of most to least waterproof. This evaluation was done by a simple visual exam of the contour feathers, then a visual exam of the down, and finally by misting the feathers repeatedly with tap water and observing the amount of water and time necessary to wet the feathers.
Notes
1Tri-State’s charter precludes the use of living animals in experiments that may harm them. The feathers used in this study were plucked from the carcasses of six snow geese, all of which had been either received dead on arrival at Tri-State or were euthanized on arrival due to the extent of their traumatic injuries. None had any evidence of oil contamination.
2All carcasses used for the final wash and evaluation were obtained in a similar manner and had no prior evidence of oil contamination.