ARTICLE
Hacked kestrels (Falco tinnunculus) show similar patterns of post-fledging food dependency as wild reared birds: implications for best practice in release management of orphaned raptors
Nathan Thavarajah1 & Miriam Fenkes2
1Wildvogel-Pflegestation, Kirchwald, Germany; 2School of Sciences, Bath Spa University, Bath, UK
Abstract
One of the challenges of wildlife rehabilitation is ensuring that rehabilitated animals have the required characteristics for survival after release. This is especially the case for orphaned animals that normally develop survival skills during a prolonged period of parental care. For raptors, this is called the post-fledging dependency period (PDP), where parents provide nutritional support to juveniles whilst they develop the physiological and behavioural characteristics required to successfully hunt prey. Orphaned raptors can be rehabilitated and released using a method termed “hacking,” a type of “soft-release” where fledglings are released from a nest box, which they learn to associate with food. This method gives the birds an opportunity to develop prey capture skills, whilst continued nutritional support is provided by rehabilitators at the box. Here, we used a hacking method to rehabilitate and release 15 orphaned kestrels (Falco tinnunculus) and observed the pattern of return to the hacking box. Of the released birds, 80% returned to the hack site for food at least once. The average number of days birds returned to the box was 12.67 ± 8.76, and there was a clear trend towards a gradual decrease in return rate (number of visits to the box per day) over time. Our observations are comparable to patterns of PDP in wild-reared kestrels and we therefore suggest that orphaned kestrels can be successfully rehabilitated in this way.
BIO
Nathan Thavarajah has a project management and research background in behavioural ecology and physiology and has contributed to several publications in these fields. After spending several years lecturing in these topics in the UK, he joined the management team of a large wildlife rehabilitation centre in Germany. Here, he worked with Miriam Fenkes and other members of the team, applying an etho-ecological approach, to focus on advancing animal welfare at the centre for animals in care as well as developing wildlife release methodologies to ensure the best chances of post-release survival of released animals. Nathan has recently taken up a post as a wildlife field worker for a government agency.
Miriam Fenkes has a research background in behavioural ecology and physiology and has contributed to several publications in these fields. After completing her PhD and spending several years lecturing in these topics in the UK, she joined the management team of a large wildlife rehabilitation centre in Germany. Here, she worked with Nathan Thavarajah and other members of the team, applying an etho-ecological approach, to focus on advancing animal welfare at the centre for animals in care as well as developing wildlife release methodologies to ensure the best chance of post-release survival of released animals. Miriam has recently taken up a post as a lecturer in wildlife ecology and conservation at Bath Spa University.
Keywords
Hacking; soft-release; raptor rehabilitation; post-release survival
Abbreviation
PDP: Post-fledging dependency period
Citation: Wildlife Rehabilitation Bulletin 2022, 40(1), 1–7, http://dx.doi.org/10.53607/wrb.v40.243
Copyright: Wildlife Rehabilitation Bulletin 2022. © 2022 N. Thavarajah and M. Fenkes. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Correspondence to: Dr. Miriam Thavarajah, School of Sciences, Bath Spa University, Newton Park, Bath, BA2 9BN. Email: m.thavarajah@bathspa.ac.uk
Accepted: 20 May 2022; Published: 7 October 2022
Disclosure statement and funding: The authors report no conflict of interest.
Wildvogel-Pflegestation Kirchwald e.V. (WPS Kirchwald) is a registered charity in Germany, and the project was funded by public donations as well as a contribution by the registered charitable trust Kurt & Maria Dohle Stiftung.
To access the supplementary material, please visit the article landing page
Introduction
The goal of wildlife rehabilitation is the successful release of rehabilitated injured and orphaned animals back into the wild. Rehabilitators therefore need to ensure that all rehabilitated animals have the capacity to perform the behaviours required for survival equal to their wild counterparts (RSPCA 2010; Miller 2012; BVZS 2016). One of the major challenges for rehabilitators is ensuring that orphaned wild animals develop the correct behavioural characteristics, so they can find food and avoid predation or injury following release. This is particularly important for predatory animals such as raptors, which need to develop well defined physiological and behavioural characteristics to enable successful prey capture. In raptors, these characteristics are developed during the PDP, a crucial life history phase, defined as the period between the first flight and independence from parents (Fox 1995; Naisbitt & Holz 2004).
Raptors face considerable challenges in acquiring food. The interaction between the predator and the prey is the product of a complex evolutionary arms race (Abrams 1986), and prey capture success rates are often low in even the most skilled of hunters (Dekker 2009). For an adult European kestrel (Falco tinnunculus), it is estimated that 40 g of live prey is required to balance its daily energy demands (Shrubb 1982), which translates into the successful capture of 2–4 small mammals per day, depending on the size of the prey caught (Masman et al. 1986). During the summer months, kestrels hunting small mammals have been observed to conduct 7.2 full strikes per hour during hunting-flight, with a success rate of 15.8% (Pettifor 1983). An adult kestrel may, therefore, need to spend several hours per day performing energetically costly hunting-flight manoeuvres to attain enough food to sustain itself (Masman et al. 1986; Masman & Klaassen 1987).
On reaching independence, juveniles will face similar nutritional demands as their adult counterparts; however, without sufficient opportunities for training to hunt prey, the birds are unlikely to be as successful (Fox 1995). Flight performance and hunting precision are developed in juveniles throughout the duration of the PDP, whilst their nutritional demands are supported by the parents. The average duration of PDP of common kestrels in Europe has been measured at 15.25 and 16 days, respectively, in two separate studies conducted in Spain (Bustamante 1994; López-Idiáquez et al. 2018) and 18 days in France (Boileau & Bretagnolle 2014), although variation can be high, likely driven by a combination of geographical location, prey availability, individual ability to learn hunting techniques and parent–offspring conflict (Shrubb 1982; Bustamante 1994; Boileau & Bretagnolle 2014; López-Idiáquez et al. 2018; Costantini & Dell’Omo 2020). During this time, there is an observed progression in their behaviour towards hunting independence. Initially, juvenile kestrels learn to hunt invertebrates, which can constitute up to one-third of their daily dietary intake (Shrubb 1982). After around 1–2 weeks, fledglings start to become more independent from their siblings, frequency of hunting activities and hunting-flight behaviours increase, and parental feeding rates decrease. It is at this point that the birds begin the trial-and-error endeavour of hunting vertebrate prey, before finally dispersing (Shrubb 1982; Bustamante 1994; Boileau & Bretagnolle 2014).
For orphaned kestrels and other raptors, the rehabilitation methods employed to return the birds back into nature may be significant in determining the likelihood of correct behavioural development, and thus, post-release survival. The preferred options may be to either return the young raptors back to the original nest or to foster them into another suitable nest (Gibson 2020). However, if these options are not available and the birds are raised in a captive setting, there are two principal methods that are subsequently used for release: “hard release” and “soft release”, which relate to the use (soft release) or non-use (hard release) of field enclosures to acclimate the birds to their release site, as well as continued food provision post-release (Mitchell et al. 2011). “Hacking” is a form of soft-release that has been used in conservation breeding and release programmes of falcons, including the peregrine falcon (Falco peregrinus; Dzialak et al. 2006), aplomado falcon (Falco femoralis septentrionalis; Brown et al. 2006), saker falcon (Falco cherrug; Dixon et al. 2020) and New Zealand falcon (Falco novaeseelandiae; Seaton 2013). In a traditional hack, fledglings are placed in a secure box at a release site, allowing them to imprint on their surroundings and associate the box with food over a period of several days. The box is then opened, allowing the birds to fledge without any human encouragement, and post-release food is provided whilst the birds learn to hunt for themselves (Brown et al. 2004; Mutch et al. 2005; Dzialak et al. 2006; Seaton 2013). It has been argued that best practice in rehabilitating orphaned raptors is achieved using the hacking method, as the released birds are afforded the opportunity to experience trial and error hunting whilst still being nutritionally supplemented (Naisbitt & Holz 2004). However, few studies have documented the use of hacking in a rehabilitation setting (Komen & Myer 1989), and therefore, measurements of what constitutes as a success in using this form of release are not well defined.
Here, we developed and deployed a hacking protocol for orphaned kestrels and observed the duration and rates that the birds utilized the site as a food source. Observations were explored in the context of PDP in wild reared birds (Bustamante 1994; Boileau & Bretagnolle 2014; López-Idiáquez et al. 2018), which may serve as a more informative proxy for rehabilitation success than common estimates of success that are based on release rates alone (Grogan & Kelly 2013).
Materials and methods
Hacking protocol
Fifteen kestrels that were admitted to a wild bird rehabilitation centre in the Eifel region, Germany, as orphans during the months of June/July 2021 were chosen for this study. All the birds were diagnosed with having no injuries. Birds were admitted at different time points in their development, so artificial broods were formed through age matching the birds as closely as possible and as early as possible in their development. The age of the birds was approximated using plumage morphology from photographs (Costantini & Dell’Omo 2020). A minimum of two and maximum of five birds were placed into a total of four broods and prepared for hacking. The birds were reared using a standardized protocol for raising raptors, with exposure to humans kept to a minimum and only being handled for routine husbandry practices. The birds were fed dead mice, with food preparation and amounts based on their stage of development (Masman et al. 1989; Naisbitt & Holz 2004; Costantini & Dell’Omo 2020). Each brood was raised at the centre until the birds were approximately 30 days, at which point they were health checked by a veterinarian and fitted with an unfastened coloured plastic leg ring, before being transported to a release site and placed into a “hack box.”
Three release sites were set up at different geographical locations, with two successive broods being released from one of the locations. The hacking procedures as well as the design and installation of the boxes were adapted from previous protocols described for the aplomado falcon and New Zealand falcon (Mutch et al. 2005; Dzialak et al. 2006; Seaton 2013), but included some design alterations and features that meant that a camera could be placed inside the box for daily health checks, and the release hatch could be opened at the front using a string pulley system, further minimizing any effect of human disturbance on the release process (see Supplementary Figs. S1–3 for details). Based on a pilot study in the previous year, the intended number of days that the birds would spend in the hack box was 7 days. However, the actual number of days that the birds spent in the hack box varied due to weather conditions, ranging 5–10 days.
The feeding regime whilst inside the hack box was two 25 g mice per bird per day. For the first 3–5 days inside the box, the ration was divided into two feeds per day, the first feed was taking place between 6:00 and 7:00 and the second between 17:00 and 18:00. Any leftover food that was accessible from the hatch at the back of the box was removed. After several days of feeding in the box, food placement was reduced to once per day, but still providing the full amount, to minimize disturbance further as the release day approached. On the final day before the planned release, the birds were given half rations in the box and two hours before sunset, 1.5 times the daily ration of frozen mice was carefully placed on the feeding platform at the front of the box, where it could be seen by the birds.
On release day, the box was opened at 5:00, approximately 30 mins before sunrise, to reduce the risk of startling the birds. Observations took place from a safe distance until all birds had left the box and were no longer visible to the observers. We did not return to the hack site on the first day of release. For the following five days post-release, 1.5 times the food ration was placed on the front of the box, divided into two feeds per day, between 5:00 and 6:00 and 17:00 and 18:00. After five days and until closure of the site, food placement was reduced to once per day, and the amount was adjusted dynamically to reflect the number of birds returning as well as theft from non-target species. A bird was assumed to have disappeared from the site if they failed to return after five consecutive days. The time frame of five days was chosen conservatively, based on return data of birds during a pilot study in the previous year, where food was provided at the hack site for the full 28 days. The longest latency between returns was 2 days. Incidentally, a similar pattern was observed in this current study, and birds that did not return to the food platform after 2 days of being observed were not seen to return thereafter. Therefore, a hack site was closed 5 days after the last bird was observed feeding there, or 28 days after the release date, whichever came first.
Return data were recorded using a field trail camera, which was located 3 m from the front of the box. It was therefore possible to ascertain the last return event for each bird and record a daily return rate (see Supplementary material for some exemplary footage).
Statistical analysis
Statistical analyses were performed using R version 4.1.1 (R Core Team 2021). Daily return rates (number of returns/day) for each bird were compared over the course of 28 days after the respective release dates for the different broods. Birds were considered to have either dispersed or met some other fate when they did not return to the site for five consecutive days and were subsequently excluded from the data set from the day after their last return. A generalized linear mixed effects model with negative binomial error distribution (MASS package [Venables & Ripley 2002] and car package [Fox & Weisberg 2019]) was fitted to analyse the relationship between the number of days post-release and number of daily returns to the release site across all released birds, with bird ID included as a random factor to account for non-independence of samples.
Results
All birds successfully left the hack box within 1.5 hours of the release hatch being opened. None of the birds were startled as the hatch was lowered, and all birds spent at least several minutes outside on the feeding platform before performing their first flight. All birds flew competently into the surrounding trees with some covering distances of over 100 m. 80% of the birds (N = 12) made at least one return to the feeding platform after leaving the box for the first time. Birds returned to the feeding platform for (mean ± S.D.) 12.67 ± 8.76 days before disappearing (range 1–27 days; n = 12), with a gradual decrease in number of birds returning over the course of the observation period (Fig. 1).
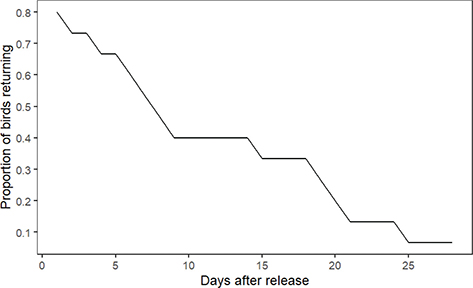
Fig. 1 Proportion of birds returning to their respective release site feeding platforms over the course of the observation period.
The birds showed a clear, but non-significant trend towards gradually decreasing the number of returns per day with increasing time post-release (generalized linear mixed effects model; χ2 = 3.7; df = 1; P = 0.054; Fig. 2).
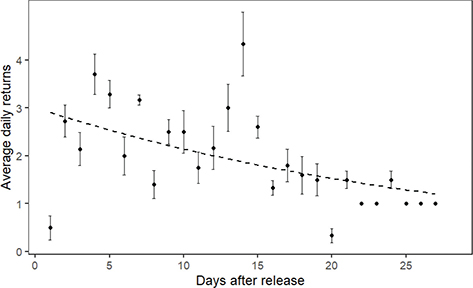
Fig. 2 Daily return rates of birds (ranging from N = 12 on day 1 to N = 1 on day 28; see also Fig. 1) over the observational period. The dashed line was fitted from a generalized linear mixed effects model, and the points and error bars show original data (mean ± S.E.M.).
Discussion
Our observations suggest that orphaned kestrels can be released into nature successfully using a hacking method. This method is well described for conservation breeding and release programmes of other falcons (Mutch et al. 2005; Brown et al. 2006; Dzialak et al. 2006), but to our knowledge, only one other study has methodically documented this type of release method in a rehabilitation setting, which incidentally was also in kestrels, the African sub-species, Falco tinnunculus rupicolus (Komen & Myer 1989).
Here, the pattern of food dependency of the hacked birds was comparable to that of wild reared common kestrels in Europe, although the average time frame of food dependency following first flight was slightly shorter than other accounts of PDPs in wild European kestrels (Bustamante 1994; Boileau & Bretagnolle 2014; López-Idiáquez et al. 2018). This may be explained by the stage of development that the hacked birds were allowed to leave the box. Kestrels naturally fledge at around 30 days (Costantini & Dell’Omo 2020) and would not begin to perform complex hunting behaviours until at least several days later, particularly with regards to the use of flight (Bustamante 1994; Boileau & Bretagnolle 2014), after which point parental food provisioning rates would also decrease (Boileau & Bretagnolle 2014). This initial lag between fledging and the development of hunting-flight behaviour in nature may be attributed to wing morphology. At fledging, the wings of kestrels are only 75% their ultimate length, even though at this point, the birds have reached their full weight capacity, with the primaries not being fully developed until around 50 days (Dijkstra et al. 1990). When compared to adults, the hunting-flight behaviours of juveniles immediately after fledging could be constrained by aerodynamic costs due to differences in wing loading, similar to that seen in sharped-shinned hawks (Accipiter striatus; Mueller et al. 1981). The birds in this study were placed in the hack box when they were at approximately 30 days and were only allowed to leave the box at a point when the wings would likely have been much closer to full growth than the point of fledging in wild birds. Therefore, it is possible that the hacked birds in this study were able to start performing hunting-flight behaviours in pursuit of prey shortly after leaving the hack box, resulting in post-release dependency on food provisions, as estimated by the number of days returning, being shorter than if we had released them earlier when full flight capabilities would have been more constrained.
Of the 15 released birds, only three (20%) failed to return to the hack site at least once, indicating that the methods used during the hacking process were sufficient to create a positive food association with the hack box. It is as difficult to speculate on the fate of the non-returners or the reason for not returning, as it is for any of the birds in this study. However, previous work on kestrels has shown a positive relationship between the probability of long-term survival (measured as recruitment) and length of PDP, where fledged kestrels were capable of long-term survival with a PDP of five days and above (Lopez-idiaquez et al. 2018). Furthermore, the minimum number of days of PDP before individuals were considered to have reached independence in other studies was three days (Boileau & Bretagnolle 2014) and eight days (Bustamante 1994). Although some mortality of fledglings is expected, particularly in the first week after fledging, subsequent weekly survival rates of fledgling raptors before independence have been found to be over 90% (Naef-Daenzer & Grüebler 2016), with observations of the mortality of fledgling kestrels before independence seen to be 9% (Bustamante 1994) and 12% (Boileau & Bretagnolle 2014). The number of days after fledging that the birds died was not specified by Bustamante (1994), but Boileau & Bretagnolle (2014) noted that all mortality during their study was observed within three days after fledging, which is consistant with Naef-Daenzer & Grüebler (2016). Therefore, the range of food dependancy days in this present study, taken in context with previous observations of PDP duration and mortality in wild fledgling kestrels, suggests that some of the birds in this study are likely to have survived at least until feeding independence. It is possible that without proper parental care during the dependency period, the hacked birds could have suffered greater predation rates than the wild reared birds observed in previous studies. However, this is unlikley to be the case, given the generally low predation rates of fledgling kestrels (9 out of 1737 predation events observed over an 8 year study period [López-Idiáquez et al. 2018]) and that adults typically do not associate with fledglings apart from during feeding (Bustamante 1994; Boileau & Bretagnolle 2014), giving limited opportunites for the tranmission of predator aversion behaviour from adults to fledglings that might otherwise be missing in the development of hacked birds.
Daily return rates showed a gradual decrease over time, implying that the birds may have become less reliant on the provisioned food source as they began to catch their own food, just as they might in nature. Boileau & Bretagnolle (2014) previously suggested that dispersal of juvenile kestrels may be under parental control, as they observed parental food provisioning to decrease throughout the PDP. But, as we show here with decreasing rates of return over time, other drivers may be influential in the dispersal process, as the hacked birds had free access to food throughout the entire hacking process. We suggest that further study should systematically investigate hacking as a rehabilitation method for orphaned kestrels and other raptors, with a special focus on post-release behaviour at the hack box, habitat use, as well as hunting and ranging behaviour, which, in the absence of a natural parent, may also shed further further light on the drivers of dispersal and other aspects of juvenile development in wild birds.
In conclusion, the success of wildlife rehabilitation is rarely judged by post-release observations of animals, but, instead, is often estimated through release rates alone. However, without observations of post-release behaviour and survival, it may not be possible to accurately reflect on and improve the success of wildlife rehabilitation efforts, and valuable resources may be wasted if post-release survival is consequently low. When taken together with observations of PDP in wild kestrels, our observations suggest that orphaned kestrels can be successfully rehabilitated through hacking. Although we cannot confirm the survival of the birds after leaving the hack site, we can be sure of our success in providing the birds with an opportunity to learn valuable hunting skills as they would in nature, without the potential energetic consequences of failure, which would not have been afforded to them if they were hard-released without any continued nutritional support. Therefore, our findings support the argument of Naisbitt & Holz (2004) that best practice in releasing orphaned raptors into nature is achieved through hacking, and unless measurements of post-release survival can be demonstrated on a species level from other forms of release, this should be the principle method used to give orphaned birds the best opportunity of survival in the wild. There are further potential benefits of employing a hacking method for the release of orphaned raptors over hard-release methods. Fundamentally, hacked birds spend less time in captivity than hard released birds that require pre-release training in an aviary for the development of flight and prey capture competencies. This reduces the risk of welfare-related factors that might be associated with the captive environment and negates the ethical concerns that are associated with feeding live prey. This relatively shorter period in captivity may also equate to a lower investment in financial support and physical resources such as food, aviary space, cleaning equipment and trained animal management personnel. Finally, we argue that rehabilitators should be critical about the specific methods that they employ to aid the transition from captivity to the wild for orphaned raptors and strive to quantify and share the outcomes of their practice, to support a culture of best practice in this sometimes-overlooked aspect of wildlife rehabilitation.
Acknowledgements
This project was made possible by the dedicated staff and volunteers at Wildvogel-Pflegestation Kirchwald e.V. (WPS Kirchwald, www.wpskirchwald.de) who helped care for the orphaned birds. The authors thank the veterinary team at AniCura Kleintierzentrum Mayen for their medical advice and expertise. The authors are indebted to the landowners who allowed the installation of the hack boxes on their properties. The authors dedicate this work and project to the memory of Dr Med. Vet. Anja Baronetzky-Mercier (1963–2020), who dedicated her life’s work to wildlife rehabilitation and protection. Her legacy lives on at WPS Kirchwald and in the many thousands of animals she saved.
References
| Abrams P.A. 1986. Is predator-prey coevolutlon an arms race? Trends in Ecology and Evolution 1(4), 108–110. doi: 10.1016/0169-5347(86)90037-6. |
| Boileau N. & Bretagnolle V. 2014. Post-fledging dependence period in the eurasian kestrel (Falco tinnunculus) in Western France. Journal of Raptor Research 48(3), 248–256. doi: 10.3356/JRR-11-70.1. |
| Brown J.L., Collopy M.W., Gott E.J., Juergens P.W., Montoya A.B. & Hunt W.G. 2006. Wild-reared aplomado falcons survive and recruit at higher rates than hacked falcons in a common environment. Biological Conservation 131(3), 453–458. doi: 10.1016/j.biocon.2006.02.021. |
| Brown J.L., Heinrich W.R., Jenny J.P. & Mutch B.D. 2004. Development of hunting behavior in hacked Aplomado Falcons. Journal of Raptor Research 38(2), 148–152. |
| Bustamante J. 1994. Behavior of colonial common kestrels (Falco tinnunculus) during the post-fledging dependence period in southwestern Spain. Journal of Raptor Research 28(2), 79–83. |
| BVZS. 2016. Good Practice Guidelines for Wildlife Rehabilitation Centres. Accessed on the internet https://www.bvzs.org/images/uploads/BVZS_Good_Practice_Guidelines_for_Wildlife_Centres_011016_.pdf on 07 June 2022 |
| Costantini D. & Dell’Omo G. 2020. The Kestrel. Cambridge: Cambridge University Press. |
| Dekker D. 2009. Hunting tactics of Peregrines and other falcons. PhD thesis, Wageningen University. Accessed on the internet https://www.researchgate.net/publication/37789436_Hunting_tactics_of_Peregrines_and_other_falcons on 07 June 2022. |
| Dijkstra C., Bult A., Bijlsma S., Daan S., Meijer T. & Zijlstra M. 1990. Brood size manipulations in the kestrel (Falco tinnunculus): effects on offspring and parent survival. The Journal of Animal Ecology 59(1), 269. doi: 10.2307/5172. |
| Dixon A., Ragyov D., Izquierdo D., Weeks D., Rahman M.L. & Klisurov I. 2020. Movement and survival of captive-bred saker falcons falco cherrug released by wild hacking: implications for reintroduction management. Acta Ornithologica 54(2), 157–170. doi: 10.3161/00016454AO2019.54.2.003. |
| Dzialak M.R., Lacki M.J., Carter K.M., Huie K. & Cox J.J. 2006. An assessment of raptor hacking during a reintroduction. Wildlife Society Bulletin 34(2), 542–547. doi: 10.2193/0091-7648(2006)34[542:aaorhd]2.0.co;2. |
| Fox J. & Weisberg S. 2019. An {R} companion to applied regression (3rd ed.). Thousand Oaks, CA: Sage. Accessed on the internet https://socialsciences.mcmaster.ca/jfox/Books/Companion/ |
| Fox N. 1995. Understanding the bird of prey. Surrey: Hancock House Publishers LTD. |
| Gibson M.C. 2020. Eagles. In R.S. Duerr & L.J. Gage (eds.), Handrearing birds (2nd ed., pp. 345–362). Hoboken, NJ: John Wiley & Sons. |
| Grogan A. & Kelly A. 2013. A review of RSPCA research into wildlife rehabilitation. Veterinary Record. doi: 10.1136/vr.101139. |
| Komen J. & Myer E. 1989. Observations on post-fledging dependence of kestrels (Falco tinnunculus rupicolus) in an urban environment. Journal of Raptor Research 23(3), 94–98. |
| López-Idiáquez D., Vergara P., Fargallo J.A. & Martínez-Padilla J. 2018. Providing longer post-fledging periods increases offspring survival at the expense of future fecundity. PLoS One 13(9), p. e0203152. doi: 10.1371/journal.pone.0203152. |
| Masman D., Dijkstra C., Daan S. & Bult A. 1989. Energetic limitation of avian parental effort: field experiments in the kestrel (Falco tinnunculus). Journal of Evolutionary Biology 2(6), 435–455. doi: 10.1046/j.1420-9101.1989.2060435.x. |
| Masman D., Gordijn M., Daan S. & Dijkstra C.O.R. 1986. Ecological energetics of the kestrel: field estimates of energy intake throughout the year. Ardea 74(October 1985), 24–39. |
| Masman D. & Klaassen M. 1987. Energy expenditure during free flight in trained and free-living eurasian kestrels (Falco Tinnunculus). The Auk 104(4), 603–616. doi: 10.1093/auk/104.4.603. |
| Miller, E.A. 2012. Minimum Standards for Wildlife Rehabilitation, 4th edition. National Wildlife Rehabilitators Association, St. Cloud, MN. Available at https://theiwrc.org/wp-content/uploads/2011/05/Standards-4th-Ed-2012-final.pdf on 07 June 2022] |
| Mitchell A.M., Wellicome T.I., Brodie D. & Cheng K.M. 2011. Captive-reared burrowing owls show higher site-affinity, survival, and reproductive performance when reintroduced using a soft-release. Biological Conservation 144(5), 1382–1391. doi: 10.1016/j.biocon.2010.12.019. |
| Mueller H.C., Berger D.D. & Allez G. 1981. Age and sex differences in wing loading and other aerodynamic characteristics of sharp-shinned hawks. Wilson Bulletin 93(4), 491–499. Accessed on the internet at https://www.jstor.org/stable/4161541 on 07 June 2022 |
| Mutch, B.D., Jenny, J.P., Heinrich, W.R., Montoya, A.B. & Sandfort, C.E. (2005) The Northern Aplomado Falcon: biology, restoration, and hacking procedures. The Peregrine Fund, Inc., Boise, ID USA. |
| Naef-Daenzer B. & Grüebler M.U. 2016. Post-fledging survival of altricial birds: ecological determinants and adaptation. Journal of Field Ornithology 87(3), pp. 227–250. doi: 10.1111/jofo.12157. |
| Naisbitt R. & Holz P. 2004. Captive raptore management and rehabiliation. Surrey: Hancock House Publishers LTD. |
| Pettifor R.A. 1983. Seasonal variation, and associated energetic implications, in the hunting behaviour of the kestrel. Bird Study 30(3), 201–206. doi: 10.1080/00063658309476797. |
| R Core Team. 2021. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Accessed on the internet at https://www.r-project.org/ on 07 June 2022 |
| RSPCA. 2010. Establishment standards for wildlife rehabiliation. West Sussex. Accessed on the internet at https://science.rspca.org.uk/sciencegroup/wildlife/reportsandresources/rehabilitation/standards on 07 June 2022 |
| Seaton R. 2013. Hack-releasing New Zealand falcons: a best-practice management guide. Wingspan Birds of Prey Trust Report. Accessed on the internet at https://wingspan.co.nz/PDF/hack_release_protocol.pdf on 07 June 2022 |
| Shrubb M. 1982. The hunting behaviour of some farmland kestrels. Bird Study 29(2), 121–128. doi: 10.1080/00063658209476746. |
| Venables W.N. & Ripley B.D. 2002. Modern applied statistics with S (4th ed.). New York: Springer. |